Salt of triazole-pyridazine derivative
A technology of triazolo and pyridazine, applied in the field of medicinal chemistry, can solve the problems of adverse reactions, drug resistance, unsatisfactory therapeutic effect, etc., and achieve the effect of practical therapeutic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
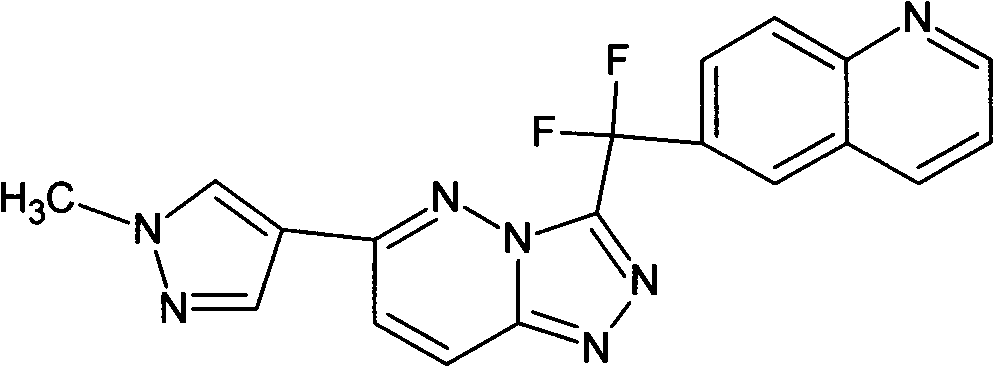
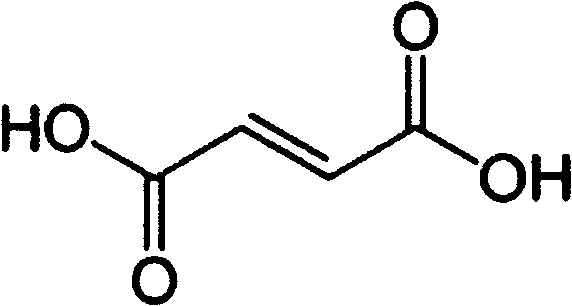
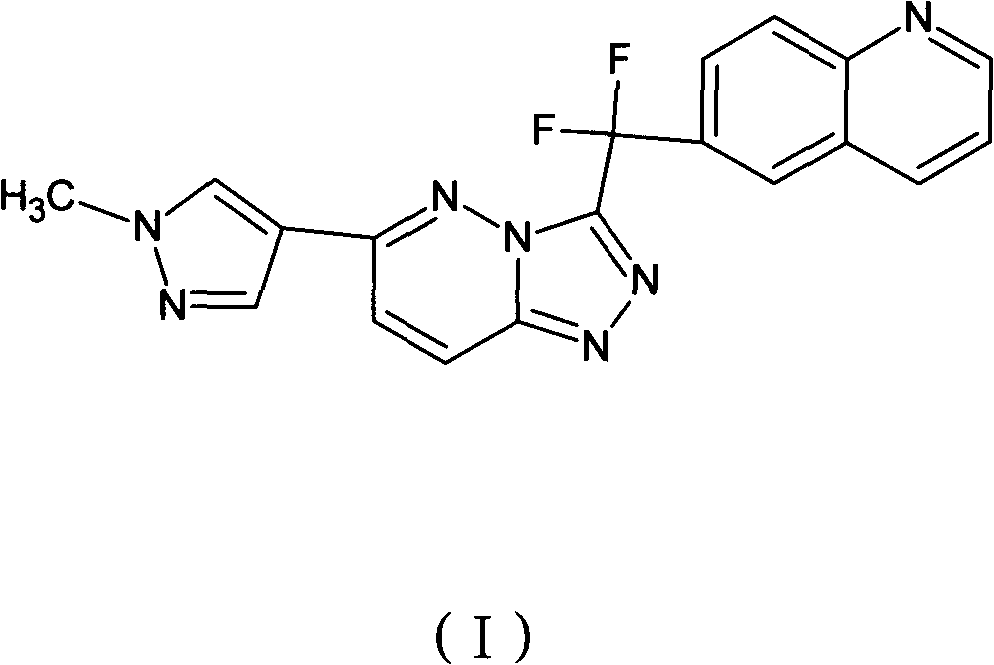
[0047] 6-{difluoro-[6-(1-methyl-1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-b]pyridazin-3-yl]formazine base}-quinoline fumarate
[0048] Compound 6-{difluoro-[6-(1-methyl-1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-b]pyridazin-3-yl ]Methyl}-quinoline 3.8 grams (0.01mol) and fumaric acid 1.17 grams (0.01mol) were dissolved in 100 milliliters of boiling ethanol. The hot solution was filtered through diatomaceous earth, then slowly cooled under gentle stirring, and stood at a temperature of 0-5°C for several hours to precipitate 6-{difluoro-[6-(1-methyl-1H-pyridine Azol-4-yl)-[1,2,4]triazolo[4,3-b]pyridazin-3-yl]methyl}-quinoline fumarate crystals, 6-{difluoro -[6-(1-Methyl-1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-b]pyridazin-3-yl]methyl}-quinoline The fumarate salt crystals were washed with ethanol and dried under vacuum at 50°C to yield 4.95 g of product.
Embodiment 2
[0050] 6-{difluoro-[6-(1-methyl-1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-b]pyridazin-3-yl]formazine base}-quinoline fumarate
[0051] Compound 6-{difluoro-[6-(1-methyl-1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-b]pyridazin-3-yl ]Methyl}-quinoline fumarate 3.8 grams and fumaric acid 1.17 grams were stirred in refluxed ethanol 100 milliliters until all solids were dissolved. Add activated carbon, filter the hot solution through diatomaceous earth, and cool down to room temperature while stirring. Standing for several hours at a temperature of 0-5°C, 6-{difluoro-[6-(1-methyl-1H-pyrazol-4-yl)-[1,2,4]triazolo [4,3-b]pyridazin-3-yl]methyl}-quinoline fumarate crystals, 6-{difluoro-[6-(1-methyl-1H-pyrazole-4- base)-[1,2,4]triazolo[4,3-b]pyridazin-3-yl]methyl}-quinoline fumarate crystals, washed with ethanol and dried under vacuum at 50°C , 4.9 g of product were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com