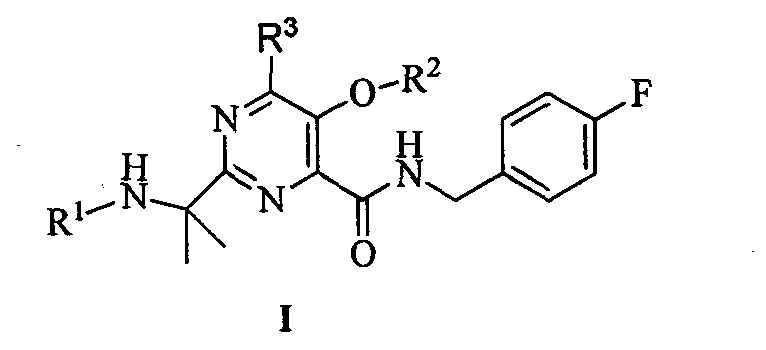
Pyrimidine amide compound and its preparation method, anti-HIV activity and anti-TMV activity
A pyrimidine amide and compound technology, which is applied to the fields of pyrimidine amide compounds and their preparation, anti-HIV activity and anti-TMV activity, can solve problems such as unsatisfactory prevention and treatment effects, and achieve good anti-HIV activity and inhibit cell infection effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
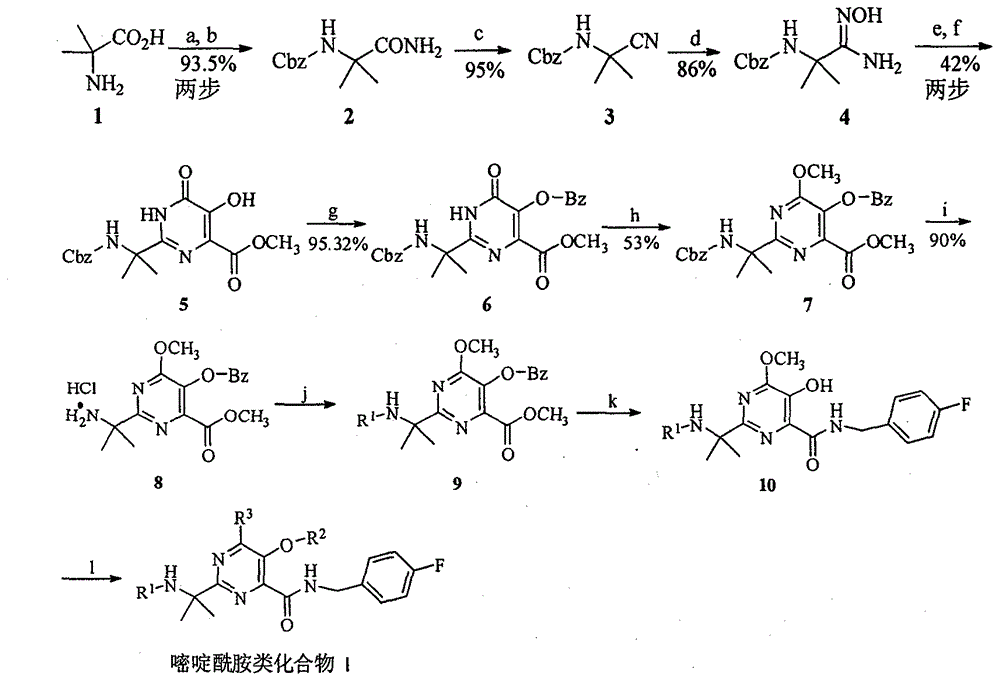
[0028]Embodiment 1: the synthesis of pyrimidine amide compound I-1, I-3 and I-12 (see equation 2 and equation 3)
[0029]
[0030] formula 3
[0031] Synthesis of Cbz-2-aminoisobutyramide 2:
[0032] In the reaction flask, add 50g 2-aminoisobutyric acid 1, dioxane, 200.97g anhydrous K 2 CO 3 , under stirring, add 124.15g benzyl chloroformate, react for 10h, add diethyl ether, separate liquid, diethyl ether phase and then use Na 2 CO 3 Solution extraction, water phase acidification, extraction, drying, precipitation to obtain 124.10 g of oily crude product Cbz-2-aminoisobutyric acid, the yield is greater than 100%, and the melting point is 65-66 °C; 1 H NMR (400MHz, CDCl 3 ): 9.24 (brs, 1H, COOH), 7.35 (s, 5H, ArH), 5.39 (brs, 1H, NH), 5.10 (s, 2H, ArCH 2 ), 1.58(s, 6H, C(CH 3 ) 2 ); directly used in the next step without further purification.
[0033] In the reaction flask, add 124.10 g of the acid crude product prepared in the previous step, THF, and 63.74 g of tr...
Embodiment 2
[0054] Embodiment 2: The chemical structural formula and physical constant of part pyrimidine amide compounds I, see Table 1:
[0055] Table 1. The chemical structural formula and physical constants of some pyrimidine amide compounds I
[0056]
[0057]
[0058]
Embodiment 3
[0059] Embodiment 3: the assay of anti-HIV activity, assay procedure is as follows:
[0060] 1 Test material:
[0061] ① TZM cells: a cell line that can respond to HIV-1 virus infection;
[0062] ② HIV-1 pseudovirus: It is assembled after transfection by the plasmid with HIV-1 envelope deletion and the plasmid providing VSVG envelope (Proc.Natl.Acad.Sci.USA 1993, 90, 8033-8037). Prepare virus stock solution for use;
[0063] ③Testing drugs: positive control drug AZT, positive control drug Raltegravir (provided by NIH), compounds I-1 to I-12.
[0064] 2 Test method
[0065] 1. Cytotoxicity test by MTT method (J. Immunol. Methods 1983, 65, 55-63): Inoculate TZM-BL cells into a 96-well plate, and spread 10 cells per well. 4 When the cells grow to 50%-60% confluence, a certain concentration of the drug to be tested is added, and the cell survival rate is detected after 48 hours. In this experiment, SRB (sulfonyl rhodamine) staining was used to detect the cell viability. Afte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com