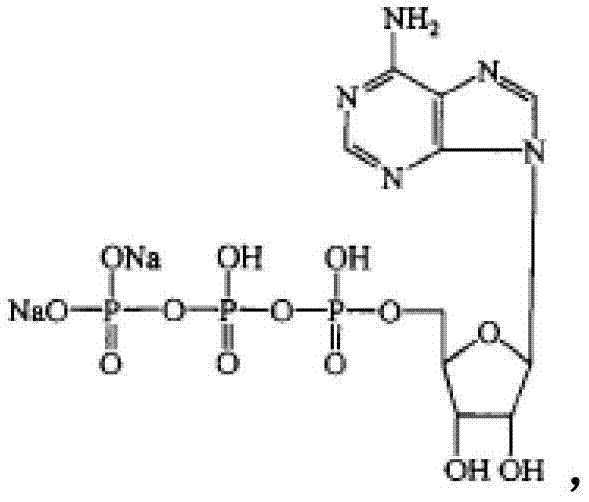
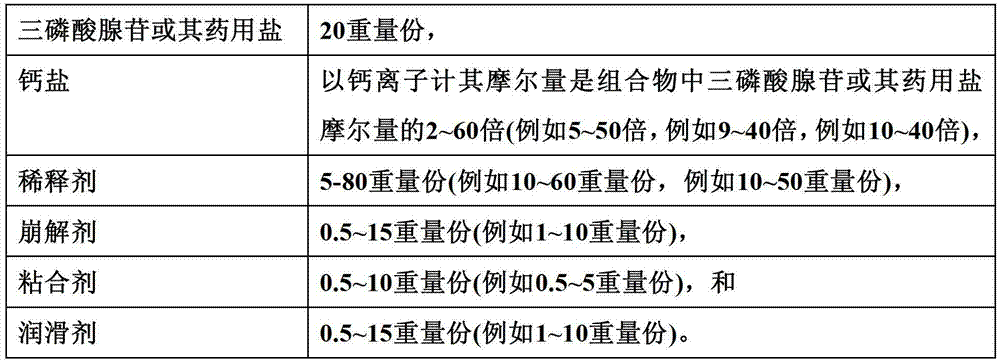
Disodium adenosine triphosphate troche medical composition
A technology of adenosine triphosphate and composition, which is applied in the direction of drug combination, pill delivery, sugar-coated pills, etc., and can solve problems such as accidental ingestion, poor operability, and increased manufacturing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] Embodiment 1: Preparation contains the pharmaceutical composition (tablet) of adenosine triphosphate disodium
[0151] Recipe (amount per tablet):
[0152] Adenosine triphosphate disodium
20mg
[0153] Sodium carboxymethyl starch
5mg
1mg
Calcium hydrogen phosphate (dihydrate)
150mg
25mg
PEG4000
7
silica
3mg
1mg
[0154] Preparation method: prepare PEG4000 with 70% ethanol to make a 5% solution as a binder, fully mix all materials except silicon dioxide and magnesium stearate, and then use the binder to wet granulate, after drying, the granules are combined with Silicon dioxide and magnesium stearate are evenly mixed, and then compressed into tablets, each containing 20 mg of disodium adenosine triphosphate.
[0155] After measurement, the weight difference of the tablet (plain tablet) is 1.4%, and the friability is reduce...
Embodiment 2
[0158] Embodiment 2: Preparation contains the pharmaceutical composition (tablet) of adenosine triphosphate disodium
[0159] Recipe (amount per tablet):
[0160] Adenosine triphosphate disodium
20mg
5mg
120mg
45mg
7
Magnesium stearate
3mg
[0161] Preparation method: prepare polyvinylpyrrolidone with 70% ethanol to make a 5% solution as a binder, fully mix all materials except magnesium stearate, and then wet granulate with binder, after drying, the granules are combined with stearin Magnesium Acid is mixed evenly, and then compressed into tablets, each containing 20 mg of adenosine triphosphate disodium.
[0162] It was determined that the weight difference of the tablet (plain tablet) was 2.0%, the friability lost 0.29% in weight, and the residual rate of adenosine triphosphate disodium was 95.5% after being sealed at room temp...
Embodiment 3
[0165] Embodiment 3: Preparation contains the pharmaceutical composition (tablet) of adenosine triphosphate disodium
[0166] Recipe (amount per tablet):
[0167] Adenosine triphosphate disodium
20mg
Low substituted hydroxypropyl cellulose
3.8mg
1.2mg
Calcium hydrogen phosphate (anhydrous)
100mg
35mg
1mg
silica
3mg
1mg
[0168] Preparation method: adopt the conventional dry granulation and tabletting process, fully mix all materials except silicon dioxide and magnesium stearate, extrude into large tablets with a dry granulator, and then crush them into 16-24 For the purpose granules, silicon dioxide and magnesium stearate are uniformly mixed with the obtained granules, and then compressed into tablets, each containing 20 mg of adenosine triphosphate disodium.
[0169] It was determined that the weight difference of the tablet (pla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com