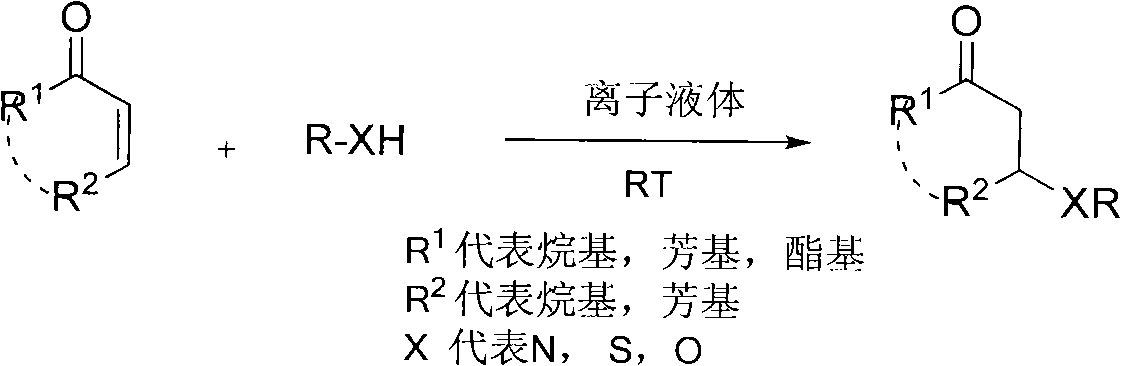
Synthetic method for beta-aminocarbonyl compound, beta-mercapto ketone and beta-alkoxy ketone
A technology of carbonyl compounds and alkoxy ketones, which is applied in the field of synthesis of β-aminocarbonyl compounds, β-mercaptoketones, and β-alkoxy ketones, achieving the effects of strong controllability, wide application range and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] At room temperature, cyclohexenone (0.5mmol, 49.0mg), ethyl carbamate (0.6mmol, 53.4mg) and [Hmim]OTs (1mL) were placed in a dry reaction bottle and reacted for 24h. After the reaction, qualitative analysis was carried out by Hewlett-Packard 6890 / 5973GC-MS and NMR. Agilent 6820 gas chromatograph was used for quantitative analysis. The yield of 3-carbamoylcyclohexanone was 75%. 1 H NMR (400MHz, CDCl 3 )δ=1.24(t, J=6.8Hz, 3H), 1.67-1.77(m, 2H), 1.99-2.03(m, 1H), 2.08-2.18(m, 1H), 2.27-2.40(m, 3H) , 2.27-2.40(m, 1H), 2.70(dd, J=4.8, 4.8Hz, 1H), 3.97(s, 1H), 4.10(t, J=2.0Hz, 2H), 5.09(d, J=7.2 Hz, 1H). 13 C NMR (100.8MHz, CDCl 3 )δ=14.6, 22.0, 31.2, 40.8, 48.0, 50.1, 60.8, 155.7, 209.0.
Embodiment 2
[0033] With example 1, catalyst used is [Hmim] HSO 4 , the yield of 3-carbamoylcyclohexanone was 70%.
Embodiment 3
[0035] With example 1, catalyst used is [Hmim] BF 4 , the yield of 3-carbamoylcyclohexanone was 71%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com