Use of ethyl pyruvate in drug preparation
A technology of ethyl pyruvate and drugs, applied in the field of pharmaceuticals, can solve problems such as reducing the incidence of disability, and achieve the effects of reducing brain tissue damage and reducing blood-brain barrier damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
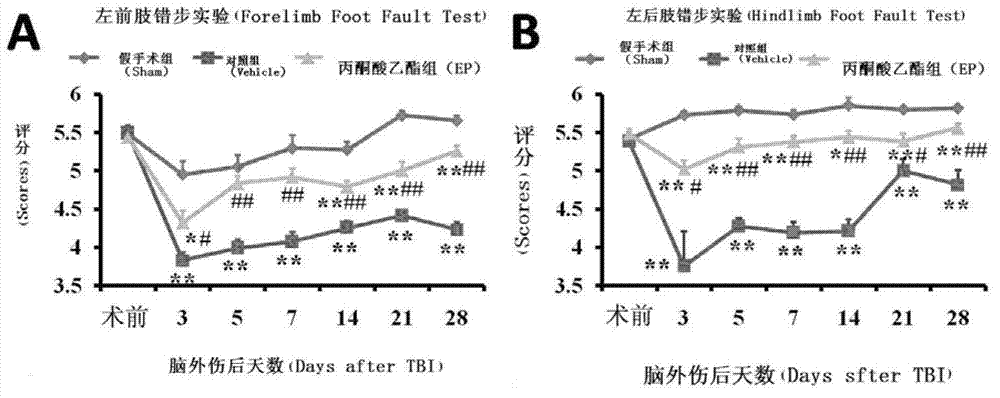
[0028] Example 1 Ethyl pyruvate improves sensorimotor recovery after traumatic brain injury
[0029] The sensorimotor ability of rats after traumatic brain injury was evaluated by step test. Animals were divided into groups: the sham group only removed the bone plate without blowing; Injection of sodium lactate Ringer injection 1ml; EP group received intraperitoneal injection of ethyl pyruvate 30mg / kg at various time points after brain trauma: three days before the brain trauma, all animals received three consecutive days of wrong step training, three times a day (per time interval 5 minutes), the third day of training was scored, and the rats that did not reach the standard were excluded (the average score was less than 5 points); the formal evaluation was performed at the set time point after the operation, and the evaluation was performed three times at the same time and place every day. It is: 0 points: completely empty, the foot falls without touching the rung, and the bo...
Embodiment 2
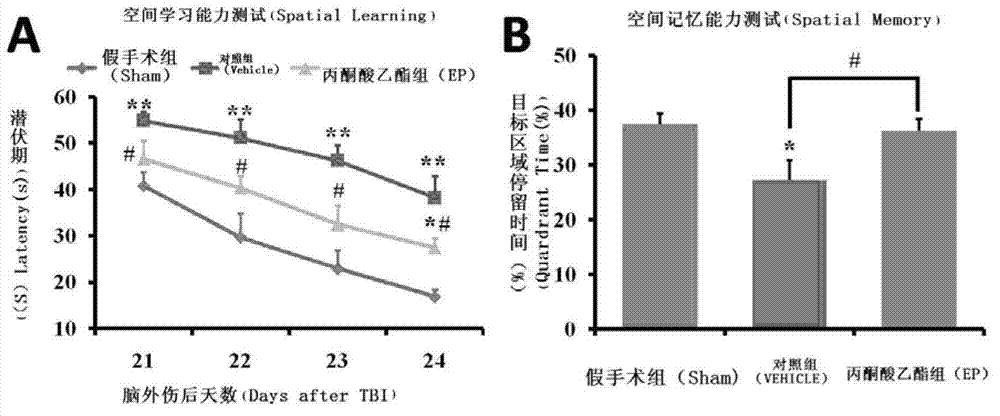
[0030] Example 2. Morris water maze task experiment of ethyl pyruvate improving spatial learning and memory ability after traumatic brain injury
[0031] The spatial learning and memory abilities of post-traumatic rats were detected by Morris water maze. Grouping: Sham group only removed the bone plate without blowing; 0, 12, 24, 36, 48, 60 hours after intraperitoneal injection of sodium lactate Ringer Injection (vehicle group) or ethyl pyruvate 30mg / kg (EP group), the rats began to receive four consecutive days of learning and training on the 21st day after traumatic brain injury, and injected into a black pool with a diameter of 1.6 meters to a depth of 35 cm Clear water with a temperature of 21±1°C divides the pool into four equal quadrants through the center of the pool, and one of the quadrants is determined as the target quadrant, and a black platform with a diameter of 10 cm is placed in the middle of it to make it 2 underwater. centimeters. Several fixed patterns were...
Embodiment 3
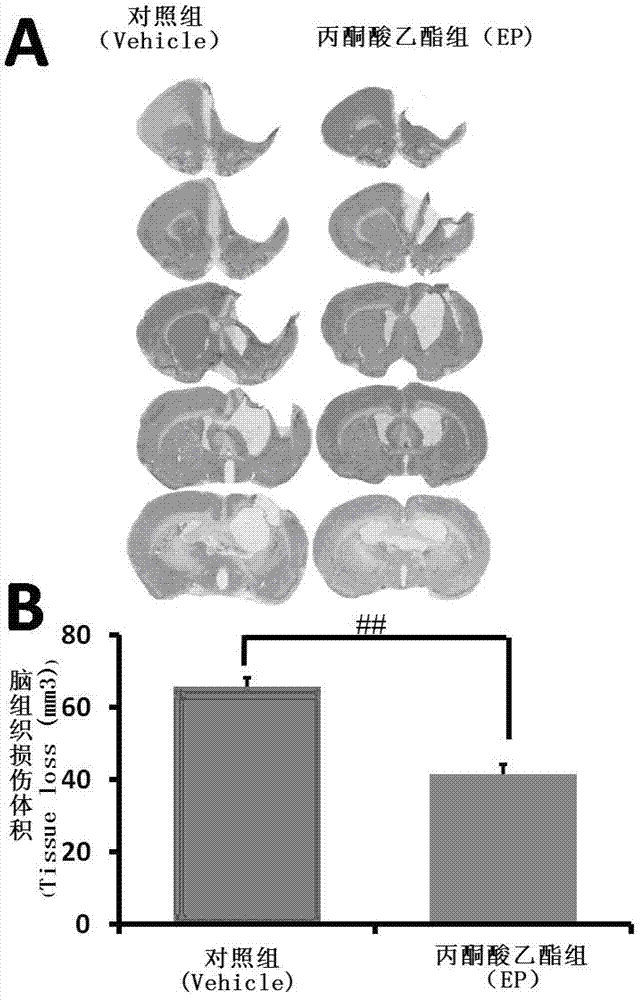
[0032] Example 3. Ethyl pyruvate reduces the volume of tissue damage in rats after traumatic brain injury
[0033] Grouping of experimental animals: Vehicle group received intraperitoneal injection of sodium lactate Ringer injection immediately, 12, 24, 36, 48, and 60 hours after brain trauma; EP group received intraperitoneal injection of ethyl pyruvate 30 mg / kg at each time point after brain trauma; At corresponding time points after trauma, the rats were anesthetized by intraperitoneal injection of 10% chloral hydrate at 360 mg / kg body weight. The animal was fixed in a supine position, and 200 ml of high-pressure sterilized normal saline was rapidly perfused through the left ventricle, followed by 100 ml of 0.1MPB (pH7.4) containing 4% paraformaldehyde, and then 100 ml of slow perfusion. Then take the brain and soak the brain tissue in 20% sucrose (pH 7.4) containing 4% paraformaldehyde and 0.1M phosphate buffer (pH 7.4) containing 30% sucrose in sequence. Perform frozen s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com