Crystalline forms of kinase inhibitors
A form, crystallization technique, applied in the field of crystalline forms of kinase inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
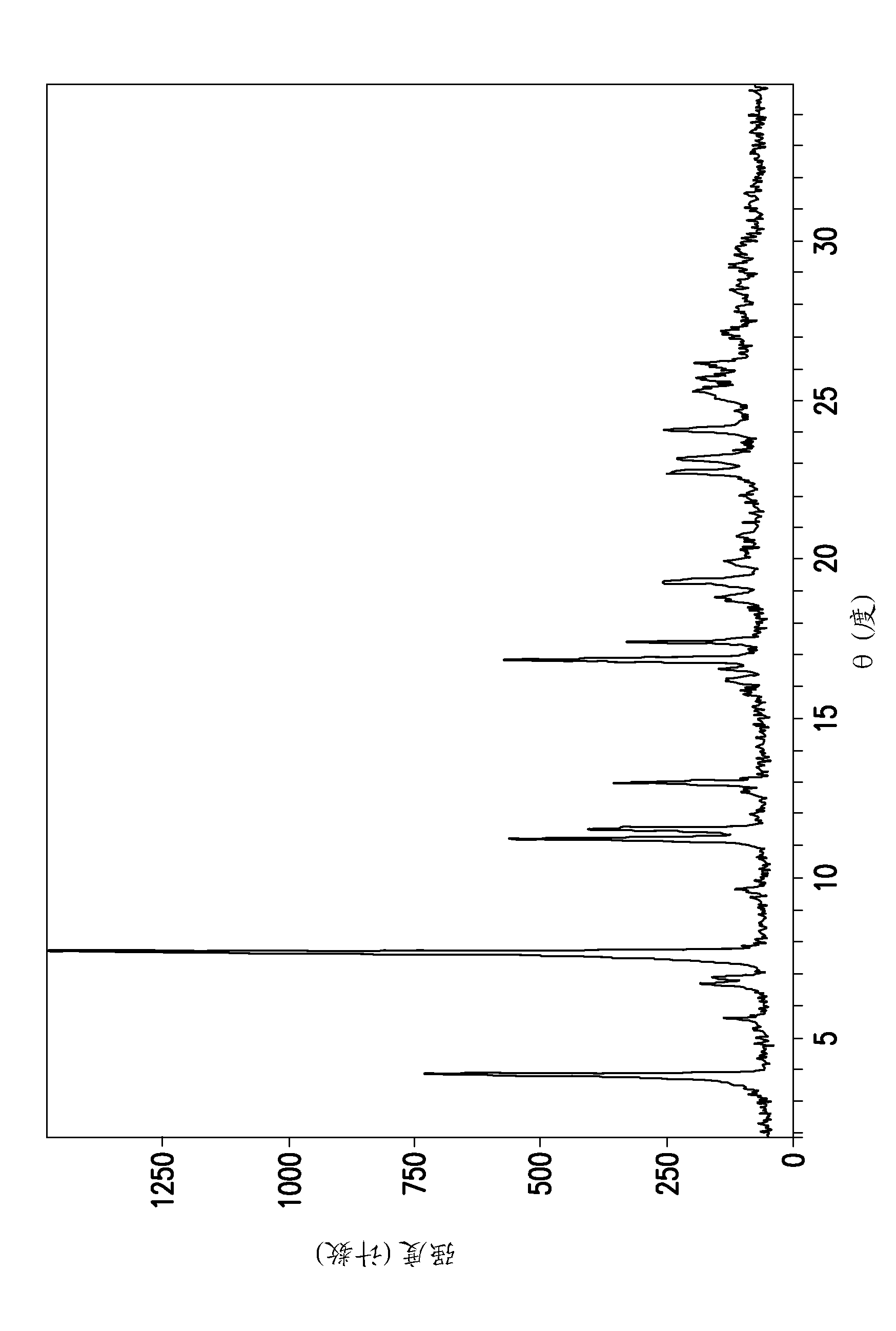
[0163] N-(4-{4-amino-7-[1-(2-hydroxyethyl)-1H-pyrazol-4-yl]thieno[3,2-c]pyridin-3-yl}phenyl )-N'-(3-fluorophenyl)urea free base solid (100 mg) was suspended in THF / water mixture (80 / 20 v / v, 500 μL). The solid was collected by centrifugation after equilibrating at ambient conditions for three days.
[0164] Table 1. PXRD peak list: N-(4-{4-amino-7-[1-(2-hydroxyethyl)-1H-pyrazol-4-yl]thieno[3,2-c]pyridine-3- Base}phenyl)-N'-(3-fluorophenyl)urea malonate type I
[0165] Peak position (° 2θ) 6.136 9.363 10.696 11.684 12.280 13.297 16.321 16.543 16.966 18.474 19.538 21.217 21.498 22.177 23.137 23.456 23.684 24.001
[0166] Example 2
Embodiment 2
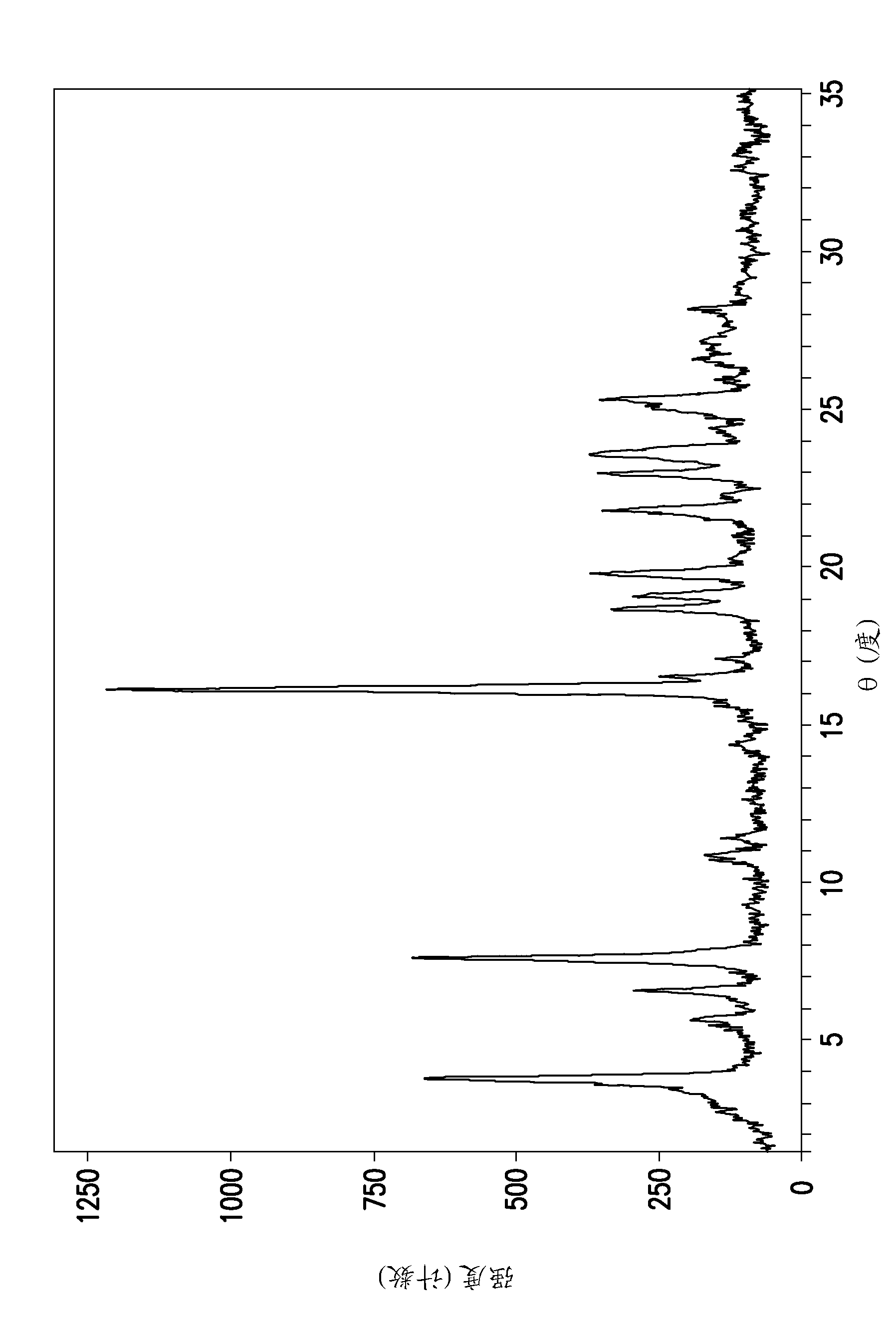
[0168] N-(4-{4-amino-7-[1-(2-hydroxyethyl)-1H-pyrazol-4-yl]thieno[3,2-c]pyridin-3-yl}phenyl )-N'-(3-fluorophenyl)urea L-bitartrate solid (30 mg) was dissolved in THF / water mixture (80 / 20 v / v, 500 μl). Single crystals were observed after six weeks of equilibration at ambient conditions.
[0169] Table 2. PXRD peak list: N-(4-{4-amino-7-[1-(2-hydroxyethyl)-1H-pyrazol-4-yl]thieno[3,2-c]pyridine- 3-yl}phenyl)-N'-(3-fluorophenyl)urea L-tartrate type II
[0170] Peak position (° 2θ) 6.205 8.879 10.277 10.644 11.958 12.436 12.763 15.933 18.479 19.181 20.581 21.155 21.382 21.745 22.504 22.861 23.855 24.500 24.916
[0171] Example 3
Embodiment 3
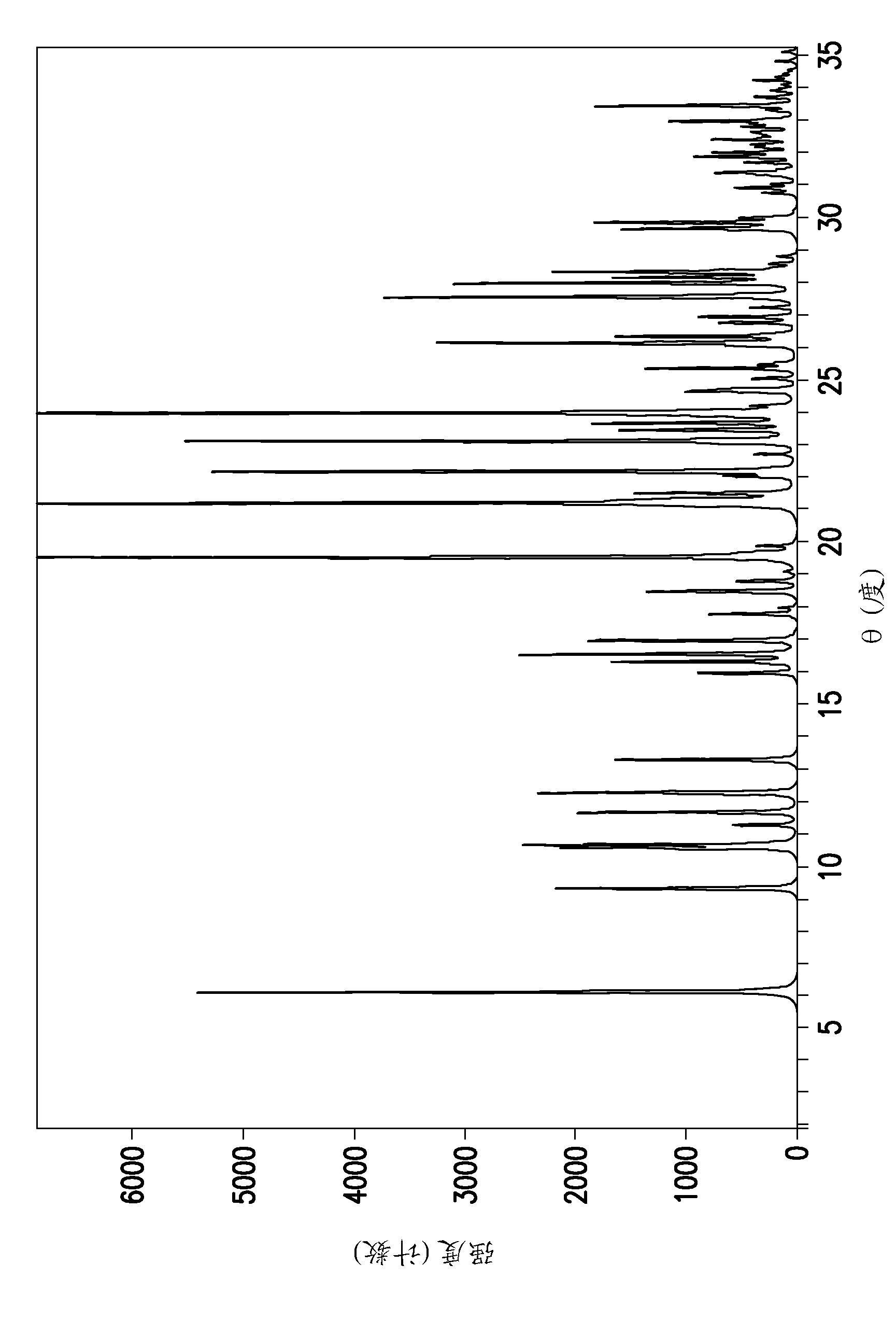
[0173] N-(4-{4-amino-7-[1-(2-hydroxyethyl)-1H-pyrazol-4-yl]thieno[3,2-c]pyridine- 3-yl}phenyl)-N'-(3-fluorophenyl)urea free base solid (27 mg) was suspended in 2-propanol (750 μl). Hydrochloric acid (60 μl, 1 N) was diluted with 2-propanol (250 μl). The hydrochloric acid solution was then slowly added to the A-968660 free base suspension under magnetic stirring at 40°C. Crystallization was observed shortly after complete addition of the HCl solution. The solid was collected by centrifugation.
[0174] Example 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com