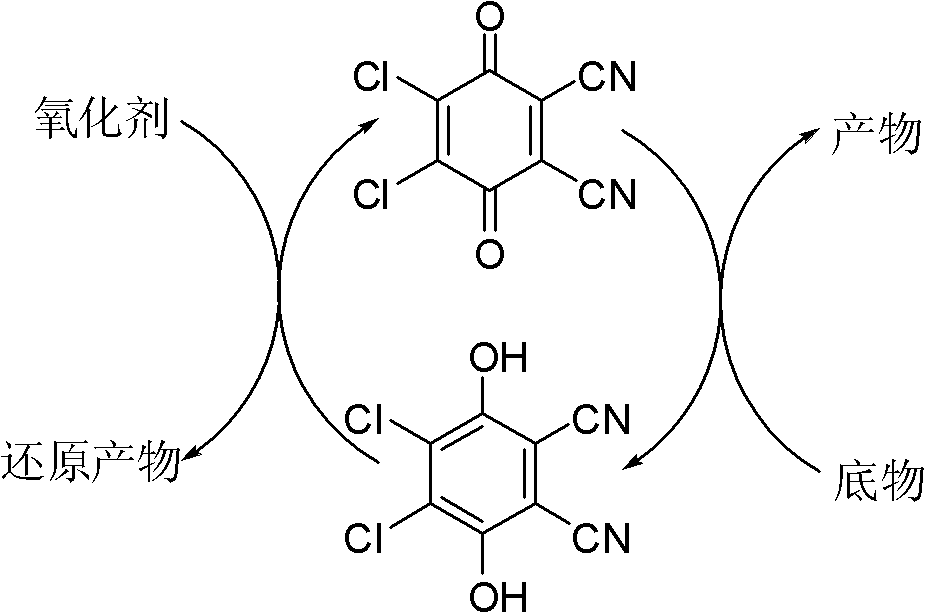
Method for preparing aldehyde or ketone by alcohol oxidation
An alcohol oxidation and oxygen technology, which is applied in the oxidation preparation of carbonyl compounds, organic chemistry, carbonyl formation/introduction, etc., can solve problems such as pollution and troublesome post-treatment, and achieve the effects of environmental friendliness, easy products and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-11
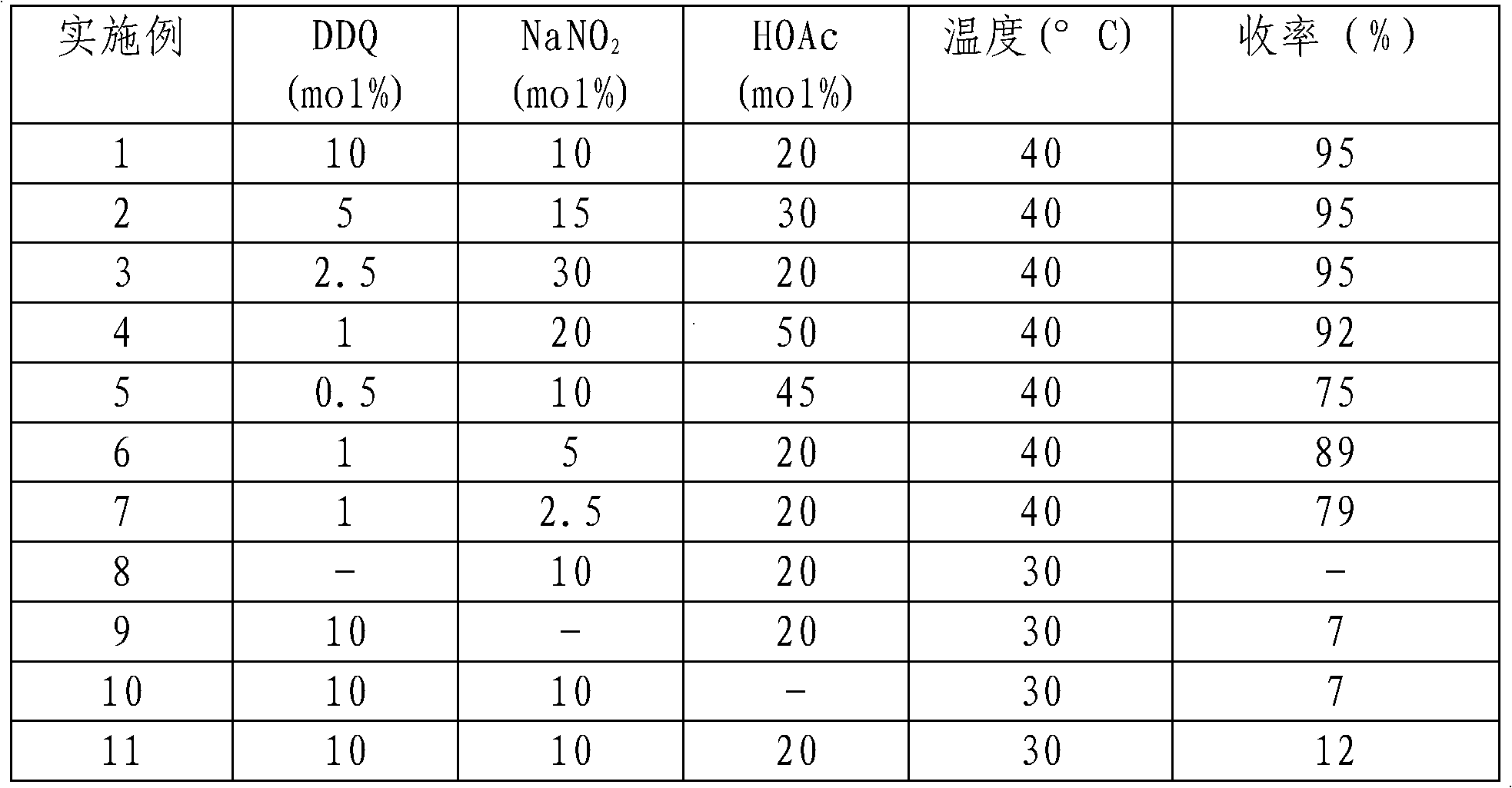
[0016] The investigation of embodiment 1-11 reaction conditions
[0017] First, we used acetic acid as an additive and cinnamyl alcohol as a model substrate to conduct a preliminary investigation on the reaction conditions, and the results are shown in Table 1
[0018] The investigation of table 1 reaction conditions
[0019]
[0020] Note: The dosage of cinnamyl alcohol is 0.1mol. Dichloromethane was used as solvent 100mL. The reaction time is 25h.
[0021] 0.1 MPaO 2 . DDQ, NaNO 2 , the amount of HOAc is relative to the amount of cinnamyl alcohol, respectively.
[0022] As can be seen from Table 1 DDQ is the most important to reaction, does not carry out without DDQ reaction, sodium nitrite and acetic acid are very important, do not add the bad product yield that reaction carries out only 7-12%. When DDQ, sodium nitrite, and acetic acid are added in a matching ratio, the product can obtain a yield greater than 90%. Of course oxygen is also very important, it is th...
Embodiment 11
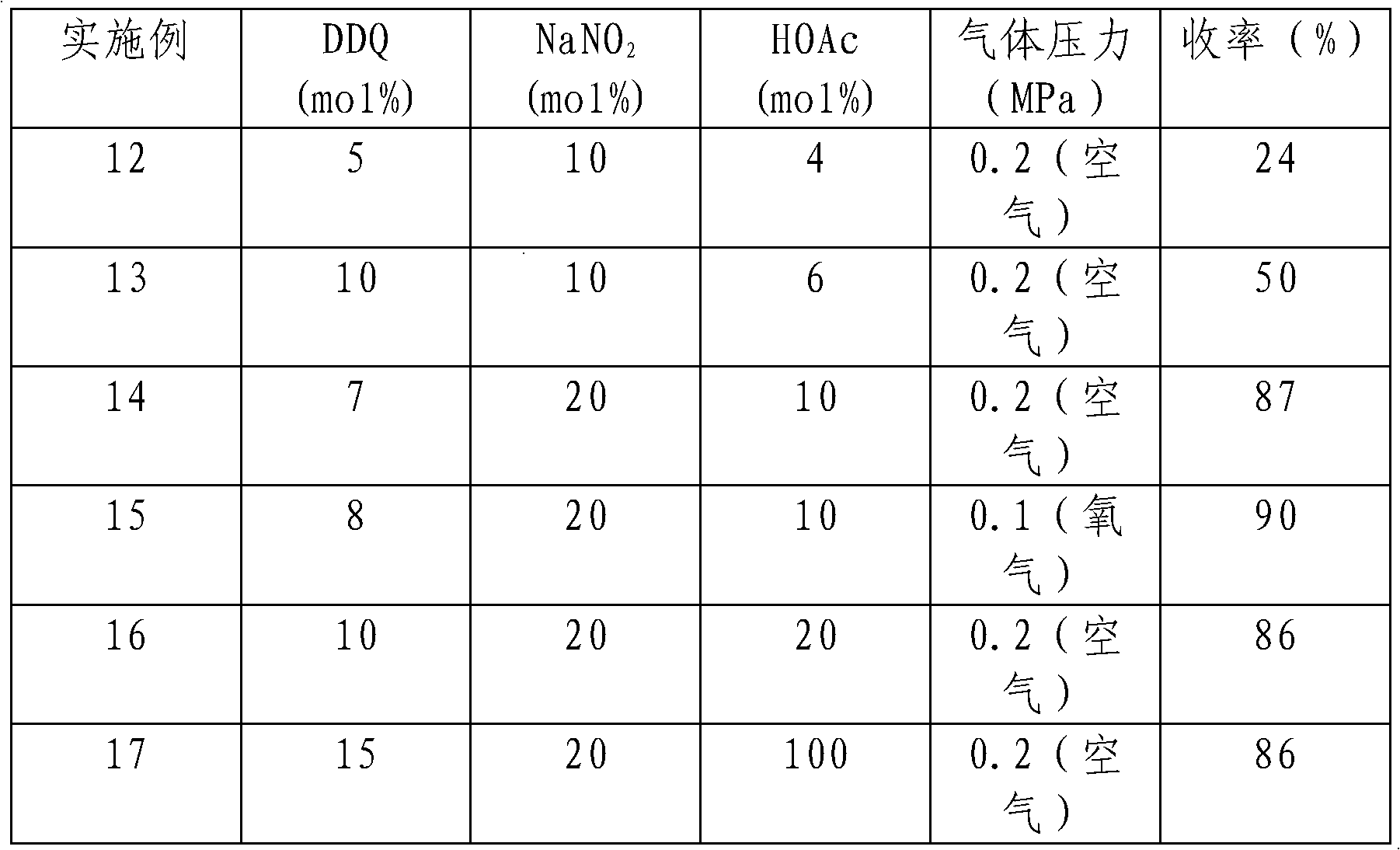
[0023] The impact of the amount of embodiment 11-acetic acid on reaction
[0024]
[0025] Note: The dosage of cinnamyl alcohol is 0.1mol. Dichloromethane was used as solvent 100mL. The reaction temperature is 35°C, and the reaction time is 25h. DDQ, NaNO 2 The amount of HOAc used is relative to the amount of cinnamyl alcohol, respectively. As seen from Table 2, the amount of acetic acid has a great impact on the reaction, and it is better when the mol ratio of acetic acid and substrate is greater than 1: 10. Oxygen as oxidant reacts faster than air as oxidant.
[0026] 18. Oxidation of cinnamyl alcohol
[0027] In a 500mL round bottom flask, add 134.18g cinnamyl alcohol, 11.35g DDQ, 69.0g sodium nitrite, 280mL toluene, 10mL acetic acid, 0.1MPaO 2 , Stir at 25°C for 36h. After the reaction was completed, the solvent was removed under reduced pressure, and the resulting mixture was dissolved in dichloromethane, washed with water, and dried to remove the dichlorometha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com