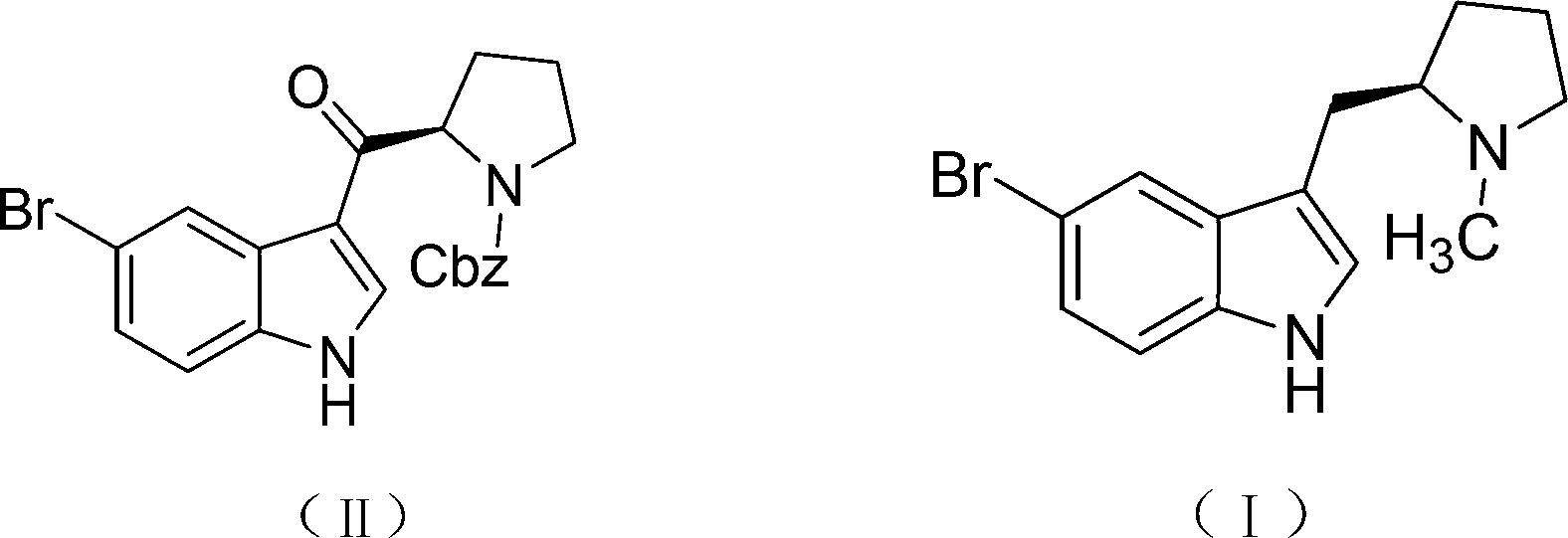
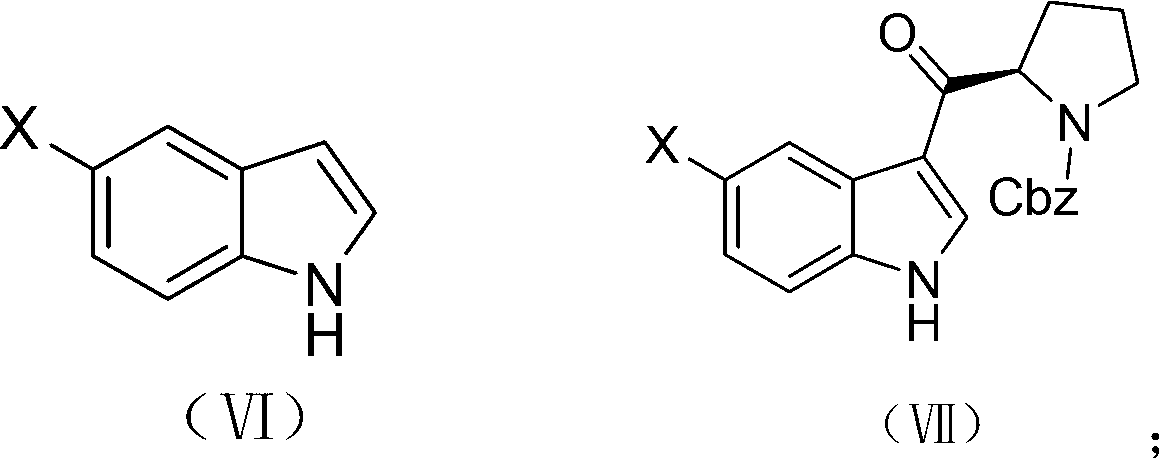
Synthetic method of (R)-5-substituted-3-(N-carbobenzoxy pyrrolidine-2-based carbonyl)-1H-benzpyrole
A technology of benzyloxycarbonylpyrrolidine and a synthesis method is applied in the field of synthesis of -5-substituted-3--1H-indole, and can solve the problems of being unsuitable for industrial mass preparation, poor operation controllability and high equipment cost, Achieving the effect of large practical application value, reduced preparation cost and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
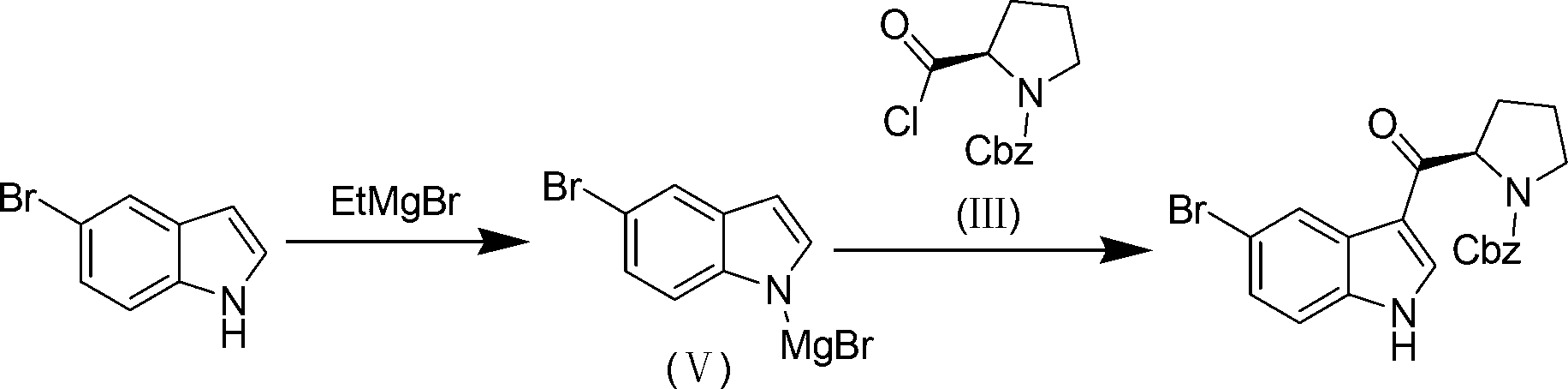
[0044] Prepare N-benzyloxycarbonyl-D-prolyl chloride according to the method in U.S. Patent No. 5,545,644, specifically as follows:
[0045] N-benzyloxycarbonyl-D-proline (0.4mol, 99.0g) was dissolved in 150mL of dichloromethane, and oxalyl chloride (0.6mol, 51.0mL) was added dropwise. Stir for 2 hours, and concentrate to dryness under reduced pressure to obtain 106.9 g of N-benzyloxycarbonyl-D-prolyl chloride as a pale yellow oil, with a yield of 100%.
Embodiment 2
[0047] 500ml three-necked bottle, add 5-bromoindole (30mmol, 5.9g), ZnCl 2 (66mmol, 9.0g) and 150mL of dichloromethane. Under ice-cooling, add 15 mL of THF solution of bromoethyl Grignard reagent (wherein, bromoethyl Grignard reagent is 2.0 mol L -1 ), and stirred for 2 hours. N-Benzyloxycarbonyl-D-prolyl chloride (33mmol, 8.8g) was added dropwise and the reaction was continued for 3 hours. Quench the reaction with saturated ammonium chloride aqueous solution, separate the layers to take the lower layer, wash with water, and saturated NaHCO 3 Washed with aqueous solution, washed with saturated NaCl aqueous solution. The upper aqueous phase was extracted with ethyl acetate, the organic layers were combined, dried and concentrated, and the crude product was recrystallized from ethyl acetate-petroleum ether (volume ratio 1:1) to obtain 10.1 g of a white solid with a yield of 79%.
[0048] white solid 1 H-NMR (DMSO-d 6 )δ:8.51(s,1H),7.63(s,1H),7.51-6.89(m,7H),5.27-5.16(m,2H)...
Embodiment 3
[0054] 500ml three-neck flask, add 5-bromoindole (5.9g, 30mmol), AlCl 3 (8.7g, 66mmol) and 150mL dichloromethane. Under ice-cooling, add 15 mL of THF solution of bromoethyl Grignard reagent (wherein, bromoethyl Grignard reagent is 2.0 mol L -1 ), and stirred for 2 hours. N-Benzyloxycarbonyl-D-prolyl chloride (33 mmol) was added dropwise and the reaction was continued for 3 hours. Quench the reaction with saturated ammonium chloride aqueous solution, separate the layers to take the lower layer, wash with water, and saturated NaHCO 3 Washed with aqueous solution, washed with saturated NaCl aqueous solution. The upper aqueous phase was extracted with ethyl acetate, the organic layers were combined, dried and concentrated, and the crude product was recrystallized from ethyl acetate-petroleum ether (volume ratio 1:1) to obtain 9.2 g of a white solid with a yield of 72%.
[0055] white solid 1 H-NMR (DMSO-d 6 )δ:8.51(s,1H),7.63(s,1H),7.51-6.89(m,7H),5.27-5.16(m,2H),5.06-4.93(m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com