High molecular weight recombinant silk or silk-like protein, and micro- or nanoscale spider-web fiber or spider-web-like fiber produced using the recombinant silk or silk-like protein
A spider silk fiber, high molecular weight technology, applied in the direction of single-component fibroin artificial filament, single-component protein rayon, fiber chemical characteristics, etc., can solve problems such as protein inhomogeneity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
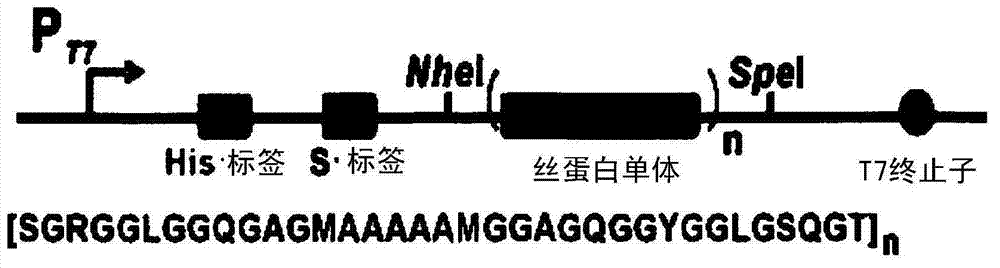
[0066]Example 1: Construction of high molecular weight silk protein expression vectors pSH32, pSH48, pSH64, pSH80 and pSH96, construction of nucleic acid sequence expression vectors encoding glycine tRNA, and preparation of recombinant silk proteins with various molecular weights.
[0067] 1-1: Construction of pSH32, pSH48, pSH64, pSH80 and pSH96.
[0068] All genetic manipulation steps were performed according to standard methods (Sambrook et al., Molecular cloning: a laboratory manual, 2nd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1989). In order to construct recombinant plasmid pSH32, plasmid pSH16a (Lee et al., Theories and Applications of Chem.Eng., 8(2): 3969, 2002) (sequence number: 13) was treated with restriction enzymes SpeI and NheI (New England Biolabs, USA) digested to obtain a 1.7kb fragment, which was treated with the restriction enzyme SpeI and connected to the dephosphorylated plasmid pSH16a, thereby obtaining the nucleic acid con...
Embodiment 2
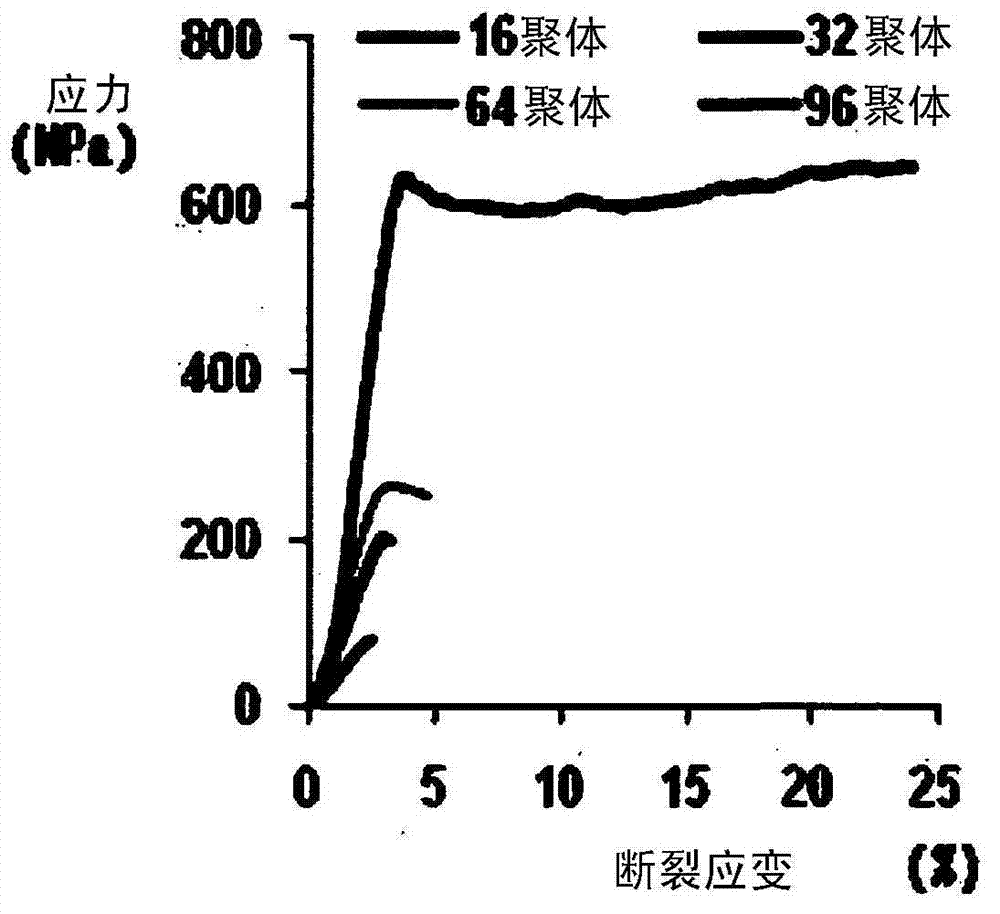
[0087] Example 2: Production of spider silk fibers by wet spinning - the influence of molecular weight on the mechanical properties of wet spinning fibers
[0088]After dissolving the recombinant silk protein prepared in Example 1-2 in the textile solvent hexafluoroisopropanol (HFIP; Sigma) to prepare the spinning solution, use a pump (KDS100; KD Scientific) at a speed of 1-2ml / hr Injection molding of spinning solution. As for the concentration of silk protein in the spinning solution, due to the relationship between solubility and viscosity, the maximum operating concentration of natural 96-mer protein is 20% (w / v), therefore, all textiles are at the concentration of silk protein in the spinning solution It is carried out under the condition of 20% (w / v). At this time, injection molding was performed by injecting the dope solution from a 1-ml Kovax syringe through a 26-G syringe needle (KoreaVaccine Co., Ltd.) into a coagulation apparatus filled with water containing 90% (v / ...
Embodiment 3
[0093] Embodiment 3: the influence of doping solution concentration on wet-spun fiber properties
[0094] In order to examine the effect of the concentration of recombinant silk protein in the spinning solution on the properties of the spun fibers, the 16-mer, 32-mer and 64-mer proteins were spun at the maximum operating concentration, and compared with Example 2.
[0095] As a result, such as Figure 5 As shown, when the concentration of 16-mer protein increased from 20% to the highest concentration of 30%, the properties of the spun fibers were significantly improved. However, when the concentration of 32-mer protein was increased from 20% to the highest concentration of 27%, the breaking strain and tensile strength of the fibers increased, but not statistically. In addition, the maximum operating concentration of 64-mer protein was 23%, and the mechanical properties at this time were not much different from those at the concentration of 20% already tested, that is, the mec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com