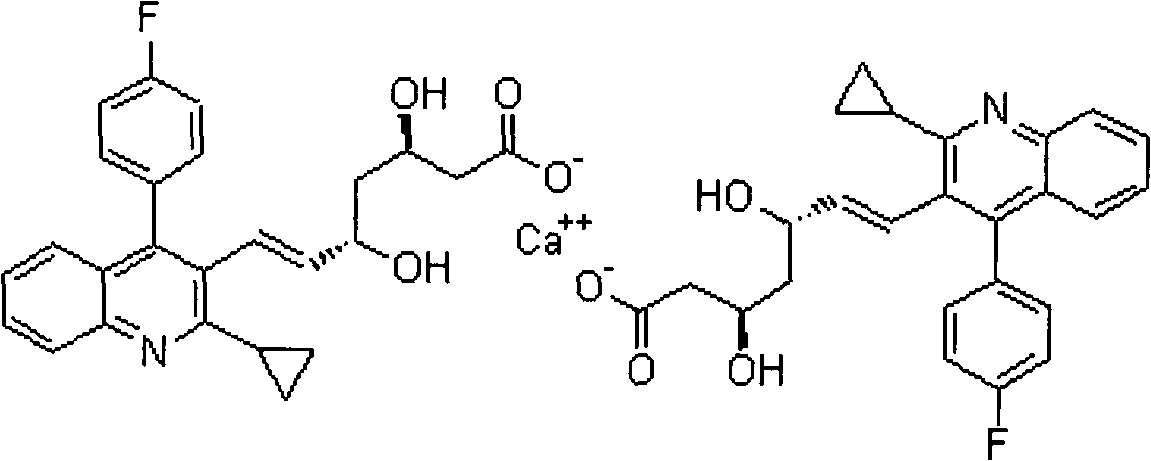
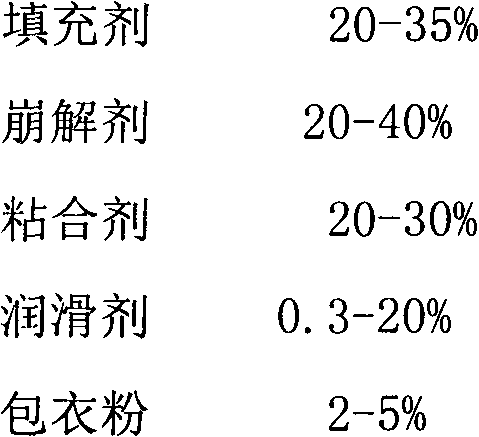
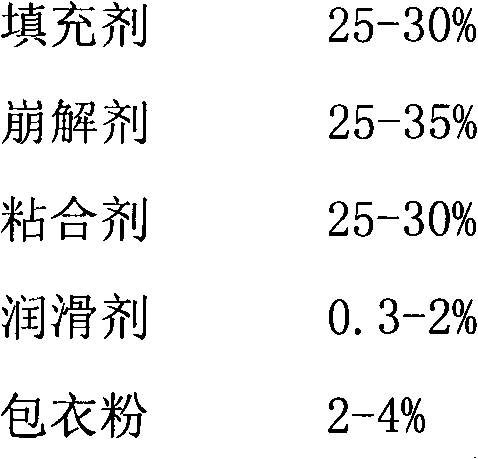
Pitavastatin calcium preparation and preparation technology
A technology of pitavastatin calcium and preparations, which is applied in the direction of pill delivery, medical preparations of non-active ingredients, sugar-coated pills, etc., and can solve problems such as poor fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] Powder direct compression process:
[0038] Mixing and granulation: first mix the main drug and excipients with good fluidity uniformly according to the method of equal volume addition, and then mix them with other excipients in a high-efficiency mixer, take samples, measure the content of intermediates, and determine the tablet weight;
[0039] Tablet compression: that is, it is compressed into tablets of suitable size on a tablet press;
[0040] Coating: Opadry is made into an aqueous dispersion of film-coating liquid, and the plain tablets are film-coated by a high-efficiency coating machine to obtain pitavastatin calcium film-coated tablets, with a weight gain of about 3%.
[0041] Wet granulation process:
[0042] Mixing and granulation: first mix the main drug and excipients with good fluidity by the method of equal volume addition, then mix them with other excipients in a high-efficiency mixer, and add povidone K 30 The aqueous solution is used as a...
Embodiment 2
[0049]
[0050] Powder direct compression process:
[0051] Mixing and granulation: first mix the main drug and excipients with good fluidity uniformly according to the method of equal volume addition, and then mix them with other excipients in a high-efficiency mixer, take samples, measure the content of intermediates, and determine the tablet weight;
[0052] Tablet compression: that is, it is compressed into tablets of suitable size on a tablet press;
[0053] Coating: Opadry is made into an aqueous dispersion of film-coating liquid, and the plain tablets are film-coated by a high-efficiency coating machine to obtain pitavastatin calcium film-coated tablets, with a weight gain of about 3%.
[0054] The film-coated tablet was accelerated for three months at 40°C and 75%, and its content was 98.0%. Related substances: isomers 0.22%, lactone 0.21%, other simple impurities 0.06%, total impurities 0.8%, all qualified .
Embodiment 3
[0056]
[0057] Powder direct compression process:
[0058] Mixing and granulation: first mix the main drug and excipients with good fluidity uniformly according to the method of equal volume addition, and then mix them with other excipients in a high-efficiency mixer, take samples, measure the content of intermediates, and determine the tablet weight;
[0059] Tablet compression: that is, it is compressed into tablets of suitable size on a tablet press;
[0060] Coating: Opadry is made into a film-coating aqueous dispersion, and the plain tablets are film-coated by a high-efficiency coating machine to obtain pitavastatin calcium film-coated tablets, with a weight gain of about 3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com