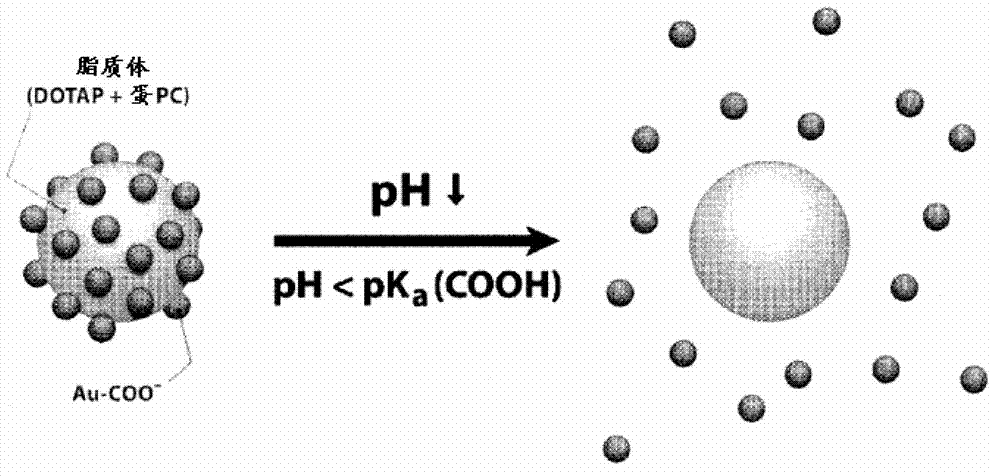
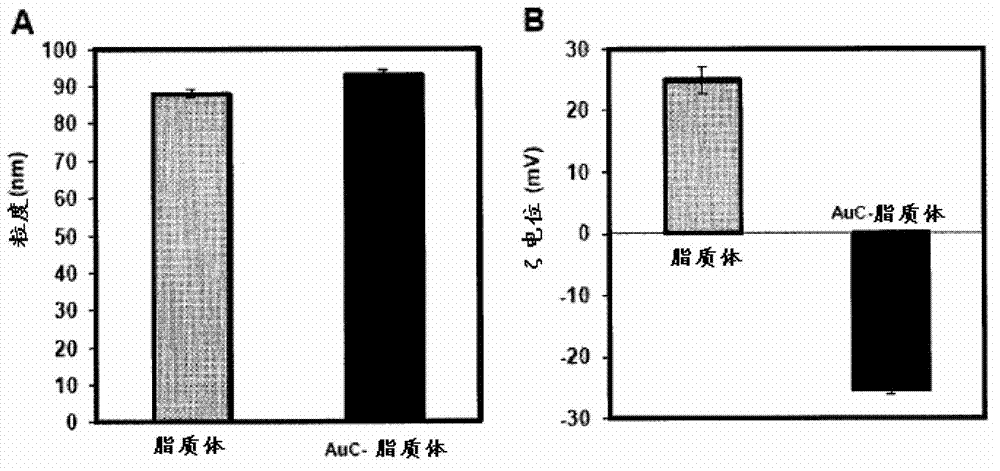
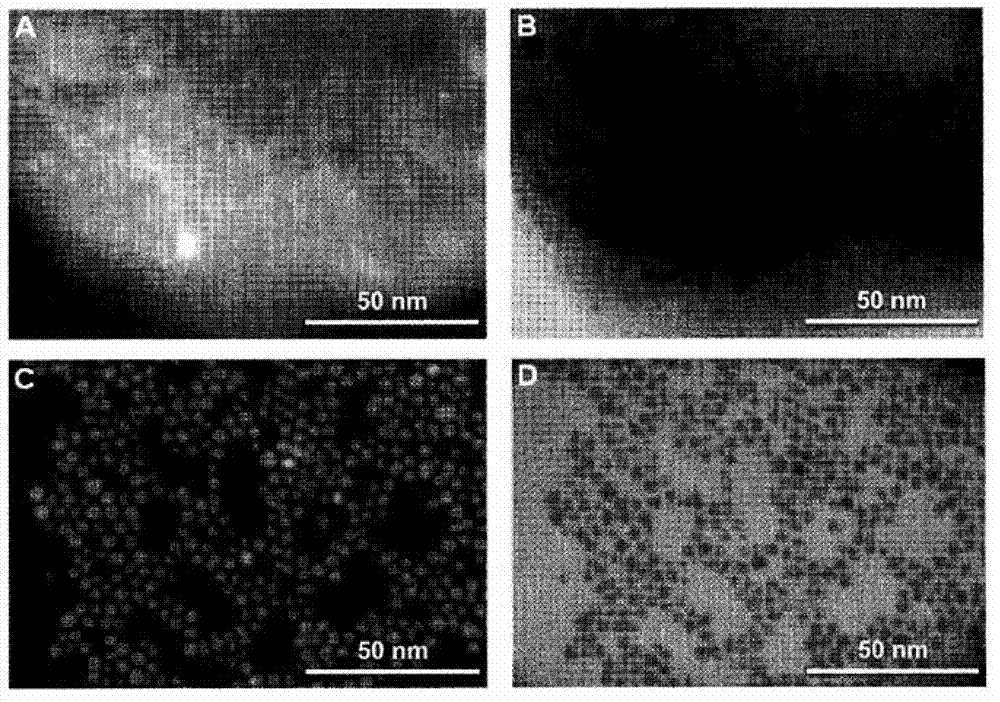
Triggered cargo release from nanoparticle stabilized liposomes
A nanoparticle and gold nanoparticle technology, applied in liposome delivery, drug combination, drug delivery, etc., can solve the problems of PEGylated liposome with less local drug delivery and less bacterial infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] The preparation of the gold nanoparticle of embodiment 1-carboxyl modification
[0096] Preparation of carboxy-modified gold nanoparticles (AuC): According to complete and detailed descriptions in other documents (Aryal, S. et al., Spectroscopic Identification of S-Au Interaction in Cysteine Capped Gold Nanoparticles.Spectrochim.ActaA2006, 63, 160-163; Patil , V. et al., Role of Particle Size in Individual and Competitive Diffusion of Carboxylic Acid Derivatized Colloidal Gold Particles in Thermally Evaporated Fatty Amine Films. Langmuir 1999, 15, 8197-8206) in sodium borohydride reduction method to prepare AuC. Briefly, at ice-cold temperature with 0.005 g NaBH 4 reduced HAuCl 4 aqueous solution (10-4M, 50 mL), thus forming bare gold nanoparticles (AuB). By mixing AuB with MPA (mercaptopropionic acid, 4×10 -4 M) Overnight incubation to carboxyl functionalize AuB. The resulting AuCs were washed 3 times through an Amicon Ultra-4 centrifugal filter with a molecular ...
Embodiment 2
[0106] Example 2 - MRSA Treatment via Liposome Pore Formation
[0107] experimental method
[0108] Materials: Hydrogenated L-α-phosphatidylcholine (egg PC) and cholesterol were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Sephadex G-75 was purchased from Fisher Scientific (Pittsburgh, PA). 8-Aminonaphthalene-1,3,6-trisulfonic acid disodium salt (ANTS) and p-xylene-bispyridinium bromide (DPX) were obtained from Invitrogen (Carlsbad, CA). Poly(ethylene glycol) methyl (Mn = 2000 Da) and Triptic Soy Broth (TSB) were purchased from Sigma Aldrich (St Louis, MO). tetrachloroauric acid (HAuCl 4 ) and sodium borohydride (NaBH 4 ) from ACROS Organics (Geel, Belgium). Chitosan-50 was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
[0109] Preparation and characterization of AuChi and AuChi-liposomes
[0110] Preparation of chitosan-modified gold nanoparticles (AuChi) by sodium borohydride reduction technique (Pornpattananangkul, D. et al., ACS Nano2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com