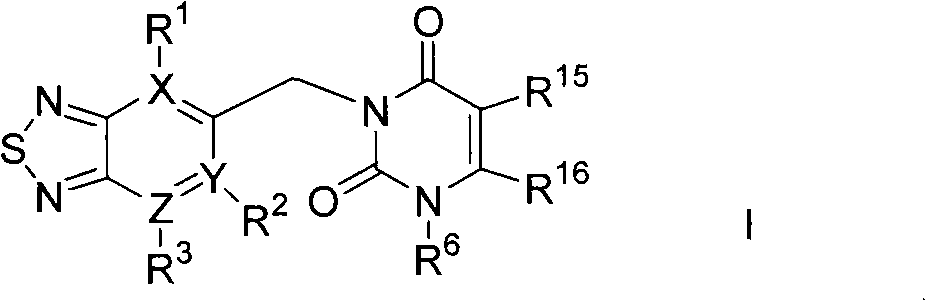
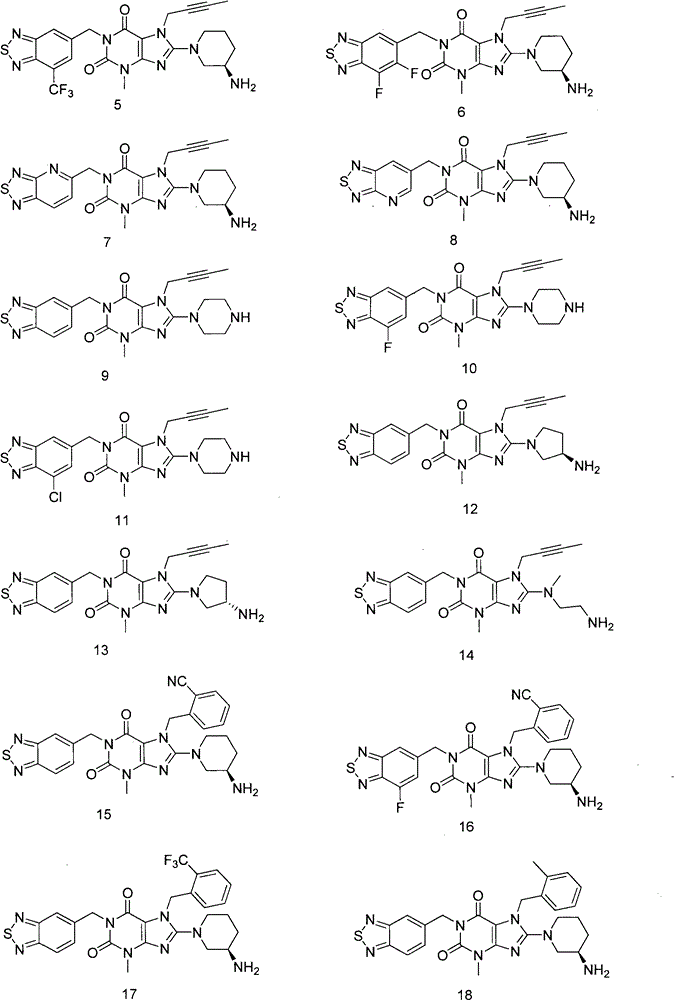
Thiadiazole derivatives dpp-iv inhibitors
A compound and the selected technology are applied in the direction of drug combination, active ingredients of heterocyclic compounds, medical preparations containing active ingredients, etc., which can solve the problems of low metabolism of linagliptin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
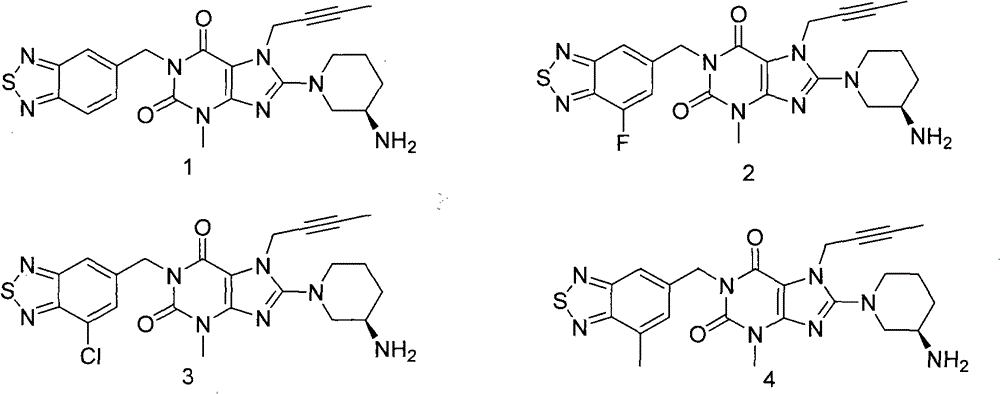
[0216] Example 1: 8-((R)-3-amino-piperidin-1-yl)-1-(benzo[1,2,5]thiadiazole-5-methyl)-7-(2- Butyn-1-yl)-3-methyl-xanthine
[0217]
[0218] General synthesis method:
[0219]
[0220] Step 1: 8-Bromo-7-(2-butyn-1-yl)-3-methyl-xanthine
[0221]
[0222] Under stirring conditions, 1-bromo-2-butyne ( 2.0g, 15mmol) and diisopropylethylamine (1.9g, 14.7mmol), reacted at 40°C for 4 hours, poured into water after cooling to room temperature, precipitated solid, filtered, washed with water, and dried in vacuum to obtain 8-Bromo-7-(2-butyn-1-yl)-3-methyl-xanthine (3.1 g, white solid), yield: 84.3%. 1 HNMR (400MHz, DMSO-d6): δ=11.31(s, 1H), 5.036-5.030(d, J=2.4Hz, 2H), 3.31(s, 3H), 1.785-1.773(t, J=2.4Hz, 3H).
[0223] Step 2: 1-(Benzo[1,2,5]thiadiazol-5-methyl)-8-bromo-7-(2-butyn-1-yl)-3-methyl-xanthine
[0224]
[0225] Under stirring conditions, 8-bromo-7-(2-butyn-1-yl)-3-methyl-xanthine (1.1g, 3.7mmol) in N,N-dimethylformamide (50mL) Add 5-bromomethylbenzo[1,2,...
Embodiment 2
[0232] Example 2: 8-((R)-3-Amino-piperidin-1-yl)-1-(7-fluorobenzo[1,2,5]thiadiazole-5-methyl)-7- (2-Butyn-1-yl)-3-methyl-xanthine
[0233]
[0234] Step 1: 1-(7-Fluorobenzo[1,2,5]thiadiazol-5-methyl)-8-bromo-7-(2-butyn-1-yl)-3-methyl- xanthine
[0235]
[0236] Referring to the method of step 2 in Example 1, replace 5-bromomethylbenzo[1,2,5]thiadiazole with 6-bromomethyl-4-fluorobenzo[1,2,5]thiadiazole , to obtain the target compound, yield: 80.5%. 1 HNMR (400MHz, CDCl3-d3): δ=7.865(s, 1H), 7.421-7.394(dd, J=1.2Hz, 10.8Hz, 1H), 5.340(s, 2H), 5.128-5.117(q, J= 2.4, 2H), 3.570 (s, 3H), 1.822-1.811 (t, J=2.4Hz, 3H).
[0237] Step 2: 8-((R)-3-tert-butoxycarbonylamino-piperidin-1-yl)-1-(7-fluorobenzo[1,2,5]thiadiazole-5-methyl) -7-(2-Butyn-1-yl)-3-methyl-xanthine
[0238]
[0239] Referring to the method of step 3 in Example 1, use 1-(7-fluorobenzo[1,2,5]thiadiazole-5-methyl)-8-bromo-7-(2-butyne-1- Base)-3-methyl-xanthine instead of 1-(benzo[1,2,5]thiadiazol-5-me...
Embodiment 3
[0243] Example 3: 8-((R)-3-amino-piperidin-1-yl)-1-(7-chlorobenzo[1,2,5]thiadiazole-5-methyl)-7- (2-Butyn-1-yl)-3-methyl-xanthine
[0244]
[0245] Step 1: 1-(7-Chlorobenzo[1,2,5]thiadiazol-5-methyl)-8-bromo-7-(2-butyn-1-yl)-3-methyl- xanthine
[0246]
[0247] With reference to the method of step 2 in Example 1, replace 5-bromomethylbenzo[1,2,5]thiadiazole with 6-bromomethyl-4-chlorobenzo[1,2,5]thiadiazole , to obtain the target compound, yield: 60.5%. 1 HNMR (400MHz, CDCl3-d3): δ=7.977-7.974 (d, J=1.2Hz, 1H), 7.797-7.794 (d, J=1.2Hz, 1H), 5.334 (s, 2H), 5.131-5.113 ( q, J=2.4, 2H), 3.572 (s, 3H), 1.824-1.812 (t, J=2.4Hz, 3H).
[0248] Step 2: 8-((R)-3-tert-butoxycarbonylamino-piperidin-1-yl)-1-(7-chlorobenzo[1,2,5]thiadiazole-5-methyl) -7-(2-Butyn-1-yl)-3-methyl-xanthine
[0249]
[0250] With reference to the method of step 3 in Example 1, use 1-(7-chlorobenzo[1,2,5]thiadiazole-5-methyl)-8-bromo-7-(2-butyne-1- Base)-3-methyl-xanthine instead of 1-(benzo[1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com