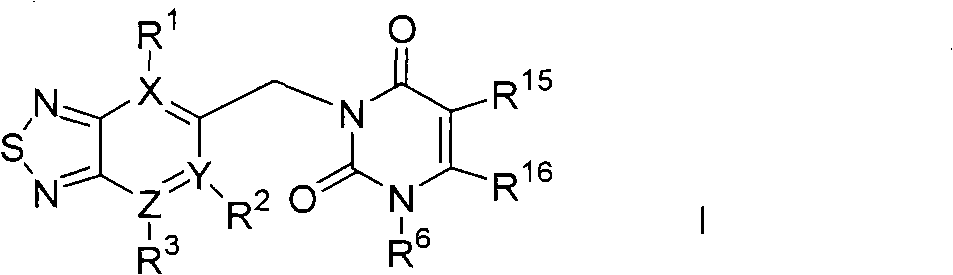
Thiadiazole derivative DPP-IV (dipeptidyl peptidase IV) inhibitor
A compound and solvate technology, applied in the field of thiadiazole derivative DPP-IV inhibitors, can solve the problem of low metabolism of linagliptin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
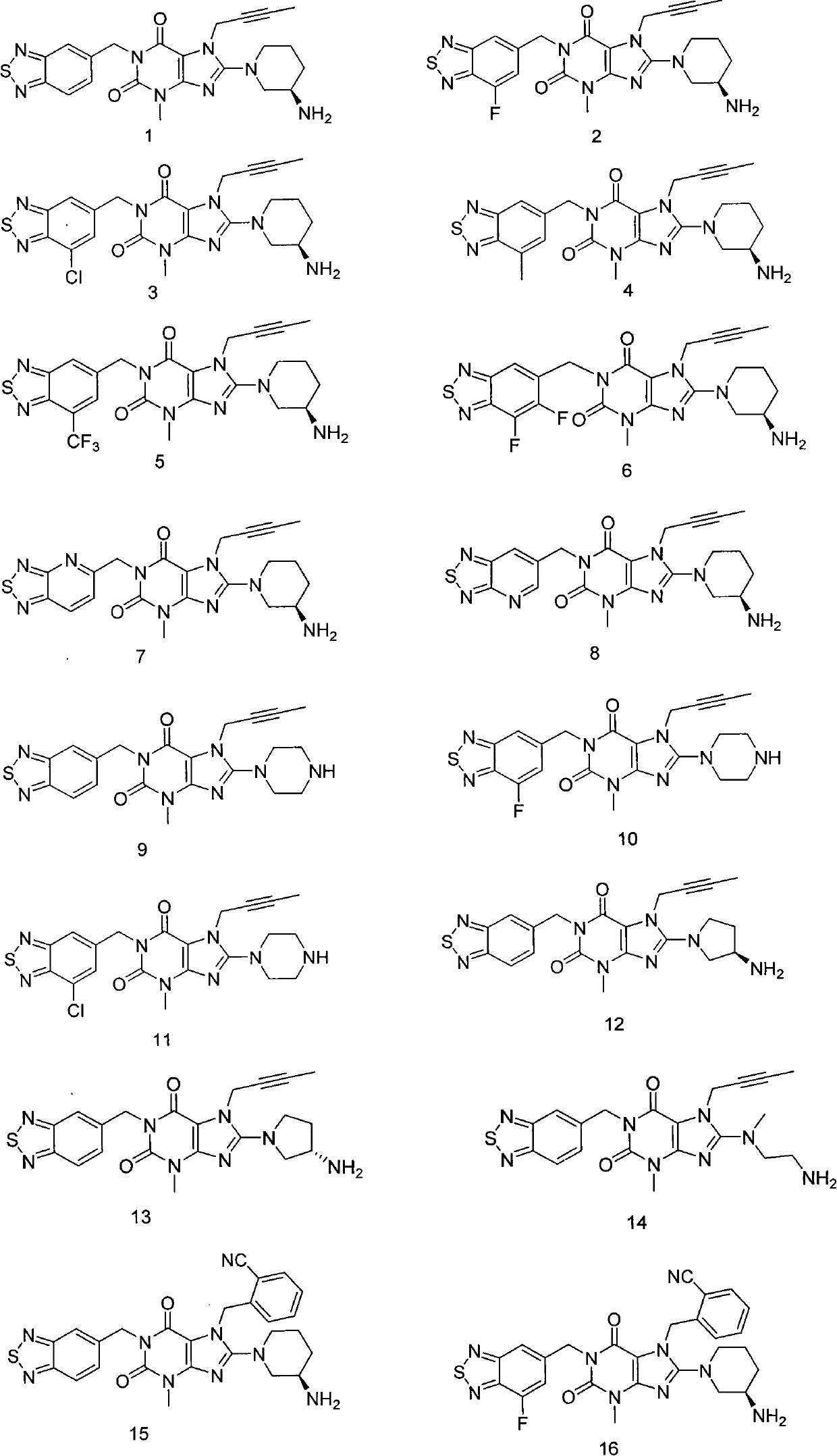
[0216] Example 1: 8-((R)-3-amino-piperidin-1-yl)-1-(benzo[1,2,5]thiadiazole-5-methyl)-7-(2- Butyn-1-yl)-3-methyl-xanthine
[0217]
[0218] General synthesis method:
[0219]
[0220] Step 1: 8-Bromo-7-(2-butyn-1-yl)-3-methyl-xanthine
[0221]
[0222] Under stirring conditions, 1-bromo-2-butyne ( 2.0g, 15mmol) and diisopropylethylamine (1.9g, 14.7mmol), reacted at 40°C for 4 hours, poured into water after cooling to room temperature, precipitated solid, filtered, washed with water, and dried in vacuum to obtain 8-Bromo-7-(2-butyn-1-yl)-3-methyl-xanthine (3.1 g, white solid), yield: 84.3%. 1 H NMR (400MHz, DMSO-d6): δ=11.31(s, 1H), 5.036-5.030(d, J=2.4Hz, 2H), 3.31(s, 3H), 1.785-1.773(t, J=2.4Hz , 3H).
[0223] Step 2: 1-(Benzo[1,2,5]thiadiazol-5-methyl)-8-bromo-7-(2-butyn-1-yl)-3-methyl-xanthine
[0224]
[0225] Under stirring conditions, 8-bromo-7-(2-butyn-1-yl)-3-methyl-xanthine (1.1g, 3.7mmol) in N,N-dimethylformamide (50mL) Add 5-bromomethylbenzo[1,...
Embodiment 2
[0232] Example 2: 8-((R)-3-Amino-piperidin-1-yl)-1-(7-fluorobenzo[1,2,5]thiadiazole-5-methyl)-7- (2-Butyn-1-yl)-3-methyl-xanthine
[0233]
[0234] Step 1: 1-(7-Fluorobenzo[1,2,5]thiadiazol-5-methyl)-8-bromo-7-(2-butyn-1-yl)-3-methyl- Xanthine
[0235]
[0236] Referring to the method of step 2 in Example 1, replace 5-bromomethylbenzo[1,2,5]thiadiazole with 6-bromomethyl-4-fluorobenzo[1,2,5]thiadiazole , to obtain the target compound, yield: 80.5%. 1 H NMR (400MHz, CDCl3-d3): δ=7.865(s, 1H), 7.421-7.394(dd, J=1.2Hz, 10.8Hz, 1H), 5.340(s, 2H), 5.128-5.117(q, J = 2.4, 2H), 3.570 (s, 3H), 1.822-1.811 (t, J = 2.4Hz, 3H).
[0237] Step 2: 8-((R)-3-tert-butoxycarbonylamino-piperidin-1-yl)-1-(7-fluorobenzo[1,2,5]thiadiazole-5-methyl) -7-(2-Butyn-1-yl)-3-methyl-xanthine
[0238]
[0239] Referring to the method of step 3 in Example 1, use 1-(7-fluorobenzo[1,2,5]thiadiazole-5-methyl)-8-bromo-7-(2-butyne-1- Base)-3-methyl-xanthine instead of 1-(benzo[1,2,5]thiadiazol-...
Embodiment 3
[0243] Example 3: 8-((R)-3-amino-piperidin-1-yl)-1-(7-chlorobenzo[1,2,5]thiadiazole-5-methyl)-7- (2-Butyn-1-yl)-3-methyl-xanthine
[0244]
[0245] Step 1: 1-(7-Chlorobenzo[1,2,5]thiadiazol-5-methyl)-8-bromo-7-(2-butyn-1-yl)-3-methyl- Xanthine
[0246]
[0247] With reference to the method of step 2 in Example 1, replace 5-bromomethylbenzo[1,2,5]thiadiazole with 6-bromomethyl-4-chlorobenzo[1,2,5]thiadiazole , to obtain the target compound, yield: 60.5%. 1 H NMR (400MHz, CDCl3-d3): δ=7.977-7.974(d, J=1.2Hz, 1H), 7.797-7.794(d, J=1.2Hz, 1H), 5.334(s, 2H), 5.131-5.113 (q, J = 2.4, 2H), 3.572 (s, 3H), 1.824-1.812 (t, J = 2.4Hz, 3H).
[0248] Step 2: 8-((R)-3-tert-butoxycarbonylamino-piperidin-1-yl)-1-(7-chlorobenzo[1,2,5]thiadiazole-5-methyl) -7-(2-Butyn-1-yl)-3-methyl-xanthine
[0249]
[0250] With reference to the method of step 3 in Example 1, use 1-(7-chlorobenzo[1,2,5]thiadiazole-5-methyl)-8-bromo-7-(2-butyne-1- Base)-3-methyl-xanthine instead of 1-(benzo[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com