Tumor vaccine of recombinant murine EPS8 gene, and preparation method and application thereof
A tumor vaccine and mouse technology, applied in the field of vaccines, can solve the problems of inability to be activated, inability to be activated, immune incompetence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
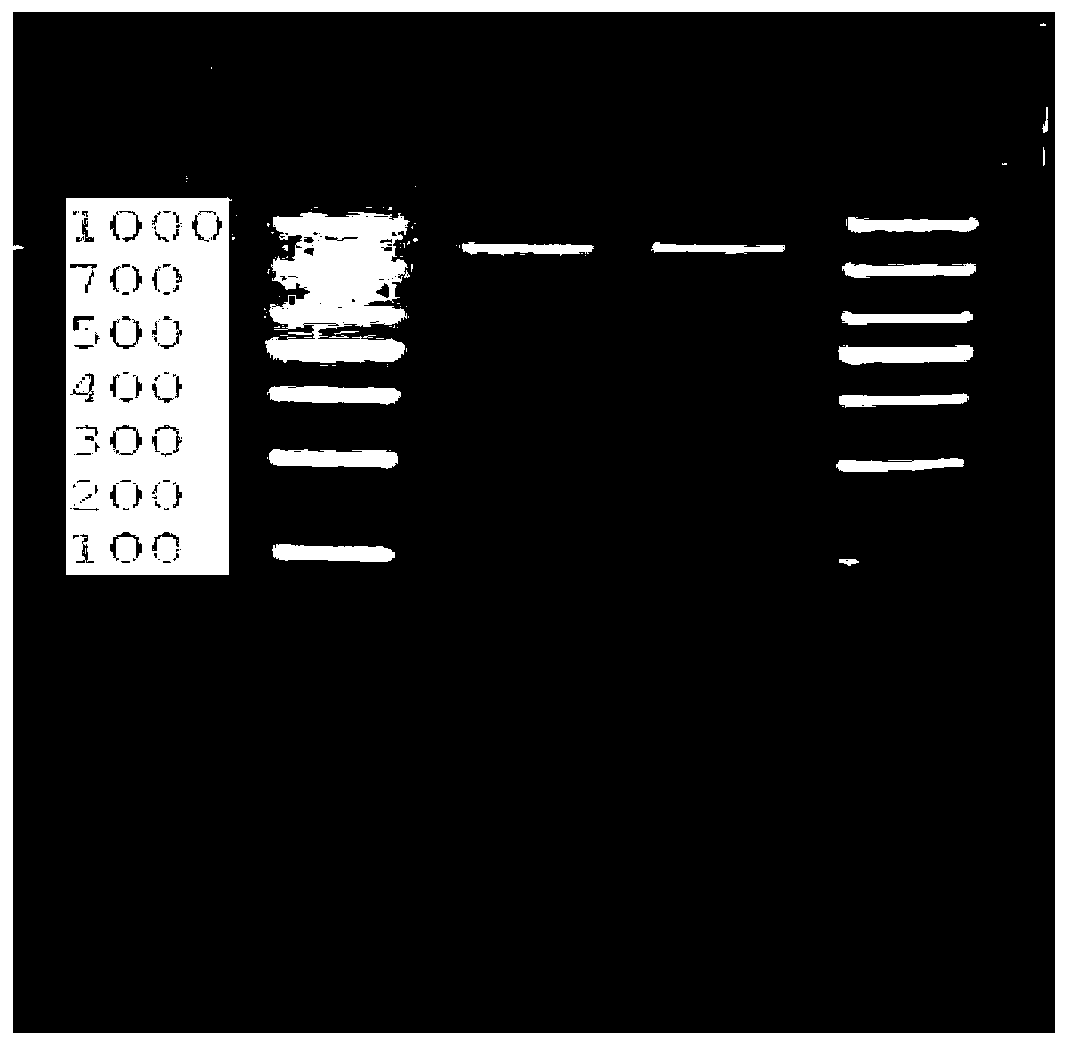
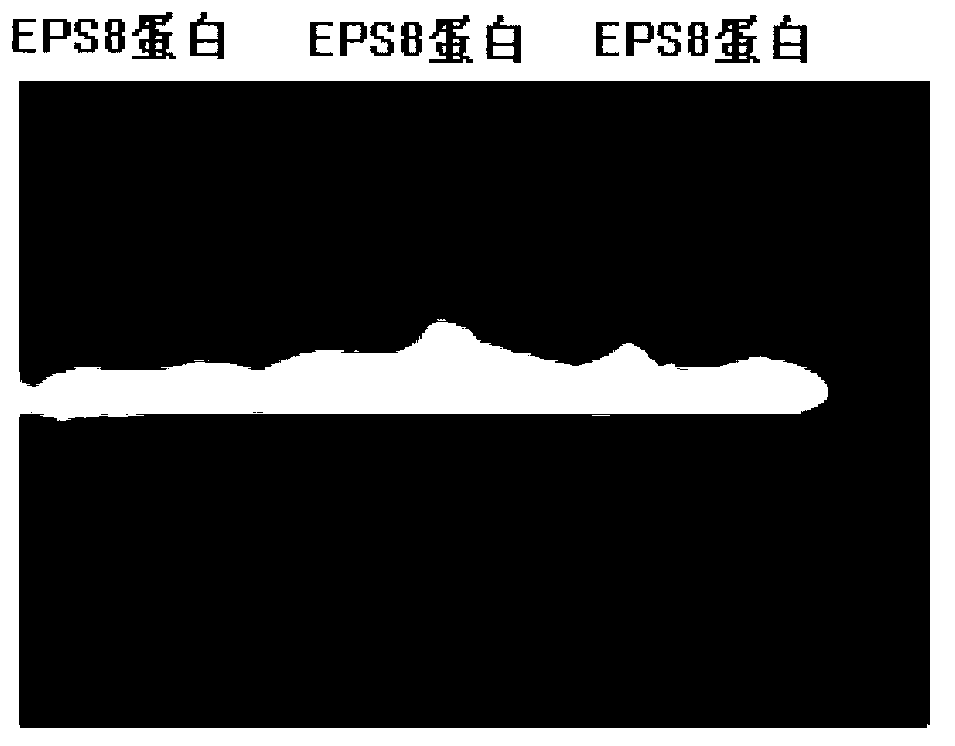
[0132] The present invention will be described in further detail below in conjunction with the accompanying drawings and embodiments.
[0133] 1. The amino acid sequence of the recombinant mouse EPS8 tumor vaccine (pET28a-rmHis-EPS8) provided by the present invention is:
[0134] Pro Pro Met Leu Asn Phe Met Gly Ala Pro Thr Glu Gln Asp Met Tyr
[0135] 1 5 10 15
[0136] Gln Leu Ala Glu Ser Val Ala Asn Ala Glu His Gln Arg Lys Gln Asp
[0137] 20 25 30
[0138] Ser Lys Arg Leu Ser Thr Glu His Ser Asn Val Ser Asp Tyr Pro Pro
[0139] 35 40 45
[0140] Ala Asp Gly Tyr Ala Tyr Ser Ser Ser Met Tyr His Arg Gly Pro His
[0141] 50 55 60
[0142] Ala Asp His Gly Glu Ala Ala Met Pro Phe Lys Ser Thr Pro Asn His
[0143] 65 70 75 80
[0144] Gln Val Asp Arg Asn Tyr Asp Ala Val Lys Thr Gln Pro Lys Lys Tyr
[0145] 85 90 95
[0146] Ala Lys Ser Lys Tyr Asp Phe Val Ala Arg Asn Ser Ser Glu Leu Ser
[0147] 100 105 110
[0148]Val...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com