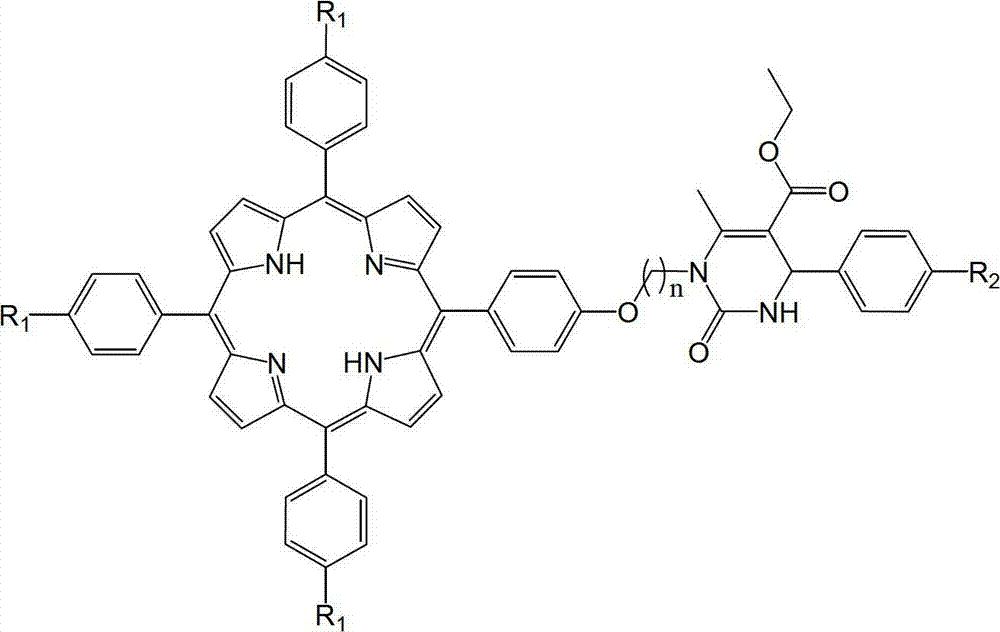
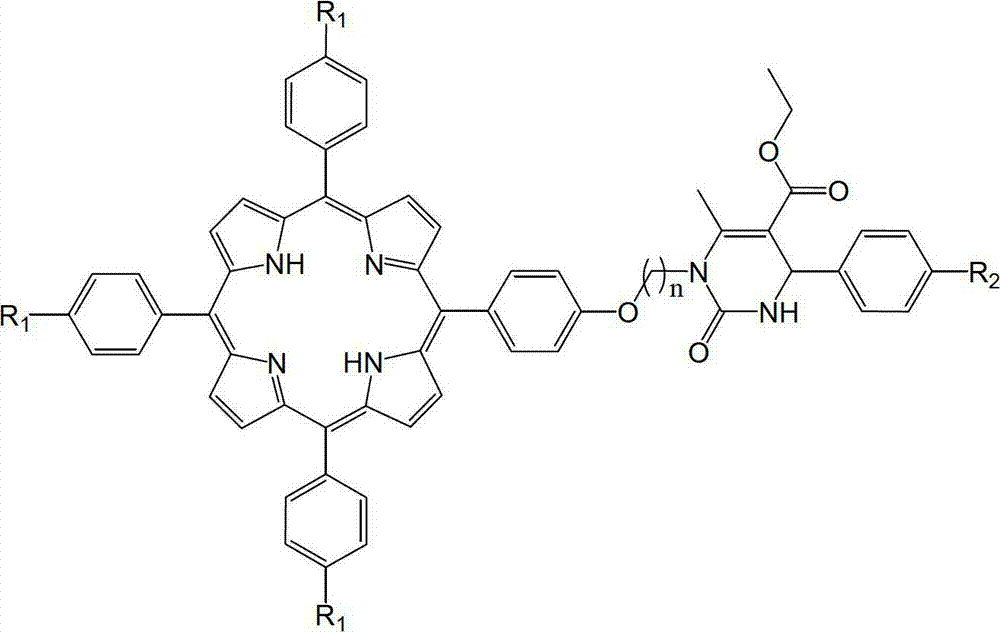
Tailed porphyrin compound modified by N1-substituted 3, 4-dihydropyrimidine-2-ketone and preparation method thereof
A technology of porphyrin compound and dihydropyrimidine, which is applied in the field of new photosensitizer and preparation, and achieves the effects of easy raw materials, simple synthesis method and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] a. Weigh 5-p-hydroxyphenyl-10,15,20-triphenylporphyrin (0.63g, 1.0mmol) in a round bottom flask, add N,N-dimethylformamide (8.0mL, 0.1mol ), so that it just dissolves completely;
[0021] b. Add N1-(4-bromobutyl)-4-phenyl-5-ethoxycarbonyl-6-methyl-3,4-dihydropyrimidin-2-one (2.0mmol ,0.79g), 5.0g of anhydrous potassium carbonate and 40mL of acetone, stirred at room temperature for 15 hours, TLC traced the complete reaction;
[0022] c. After that, the acetone was evaporated under reduced pressure, and then distilled water was added. After filtering and drying, column chromatography was performed with 300-400 mesh silica gel. First use the mixed solvent of dichloromethane and sherwood oil with a volume ratio of 2.5:1 as the eluent to elute the monohydroxyporphyrin, and then use a mixed solvent of ethyl acetate and sherwood oil with a volume ratio of 1:5 to carry out Elution, collect the third point. After precipitation and drying, a purple solid was obtained with a yi...
Embodiment 2
[0026] Using substantially the same process as in Example 1, the raw material was changed to N1-(4-bromopentyl)-4-phenyl-5-ethoxycarbonyl-6-methyl-3,4-dihydropyrimidin-2-one , the dosage was 2.5 mmol, and a purple solid was obtained with a yield of 85%.
[0027] The spectral characterization data of the product are as follows: 1 H NMR (CDCl 3 ,500MHz)δ:-2.74(s,2H,pyrrole-NH),1.17(t,3H,J=7.5Hz,ester-CH 3 ),1.58-1.97(m,6H,3×CH 2 ),2.59(s,3H,CH 3 ),3.67-3.73(m,1H,NCH 2 ),3.99-4.05(m,1H,NCH 2 ),4.11(q,2H,J=7Hz,OCH 2 ), 4.17(t,2H,J=6Hz,ester-CH 2 ),5.39(s,1H,CH),5.56(br s,1H,NH,exchanged with D 2 O),7.23(d,2H,J=8.5Hz,5-phenyl),7.27-7.34(m,5H,DHPM-phenyl),7.71-7.74(m,9H,phenyl),8.10(d,2H,5 -phenyl), 8.21 (d, 6H, phenyl), 8.84 (s, 6H, β-pyrrole), 8.89 ((s, 2H, β-pyrrole)). 13 C NMR (126MHz, CDCl 3)δ:166.21,158.92,153.64,148.69,143.54,142.28,135.65,134.60,134.49,128.77,127.92,127.72,126.70,126.33,120.19,120.11,119.99,112.77,104.78,99.99,67.99,60.27,54.16,42.70 , 29.77, 29....
Embodiment 3
[0029] Using substantially the same process as in Example 1, the raw material was changed to N1-(4-bromohexyl)-4-phenyl-5-ethoxycarbonyl-6-methyl-3,4-dihydropyrimidin-2-one, The dosage was 2.5 mmol, and a purple solid was obtained with a yield of 83%.
[0030] The spectral data of the product are as follows: 1 H NMR (CDCl 3 ,500MHz)δ:-2.74(s,2H,pyrrole-NH),1.16(t,3H,J=7Hz,ester-CH 3 ),1.59-1.93(m,8H,4×CH 2 ),2.58(s,3H,CH 3 ),3.62-3.67(m,1H,NCH 2 ),3.96-3.99(m,1H,NCH 2 ),4.09(q,2H,J=7Hz,OCH 2 ), 4.18(t,2H,J=6Hz,ester-CH 2 ),5.38(s,1H,CH),5.56(br s,1H,NH,exchangedwith D 2 O),7.22(d,2H,J=5Hz,5-phenyl),7.26-7.32(m,5H,DHPM-phenyl),7.72(br,9H,phenyl),8.09(d,2H,5-phenyl) , 8.21 (br, 6H, phenyl), 8.84 (s, 6H, β-pyrrole), 8.88 ((s, 2H, β-pyrrole)). 13 C NMR (126MHz, CDCl 3 )δ:166.21,158.98,153.53,148.74,143.55,142.29,135.65,134.60,134.44,128.75,127.90,127.71,126.70,126.33,120.23,120.11,119.98,112.77,104.71,68.08,60.24,54.15,42.72,29.92 , 29.45, 26.97, 26.72, 26.05, 16.24, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com