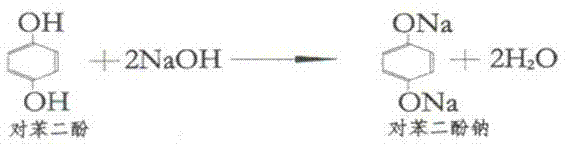Method for preparing 2-(4-methoxy phenoxy) propionic acids
A technology of oxyphenoxy and propionic acid, which is applied in the field of preparation of herbicide intermediates, can solve the problems of high cost, time-consuming, troublesome intermediate treatment, etc., and achieve the effect of low cost, less pollution and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] In order to further illustrate the technical solution of the present invention, the preferred embodiments of the present invention are described below in conjunction with examples, but it should be understood that these descriptions are only to further illustrate the features and advantages of the present invention, rather than limiting the claims of the present invention.
[0032] The purity of hydroquinone, methyl S-2-chloropropionate and sodium hydroxide used in this example are all ≥99%, and the purity of hydrochloric acid is ≥30%.
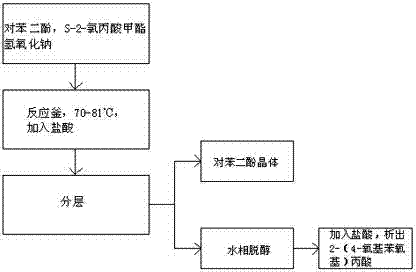
[0033] A kind of preparation method of 2-(4-oxyphenoxy group) propionic acid, comprises the following steps (such as figure 1 shown):
[0034] (1), prepare raw materials according to 183kg of hydroquinone, 202.5kg of S-2-methyl chloropropionate, 210kg of sodium hydroxide and 426kg of hydrochloric acid;
[0035] (2) Add 183kg of hydroquinone and 202.5kg of methyl S-2-chloropropionate into the reaction kettle all at once, add 100kg of so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com