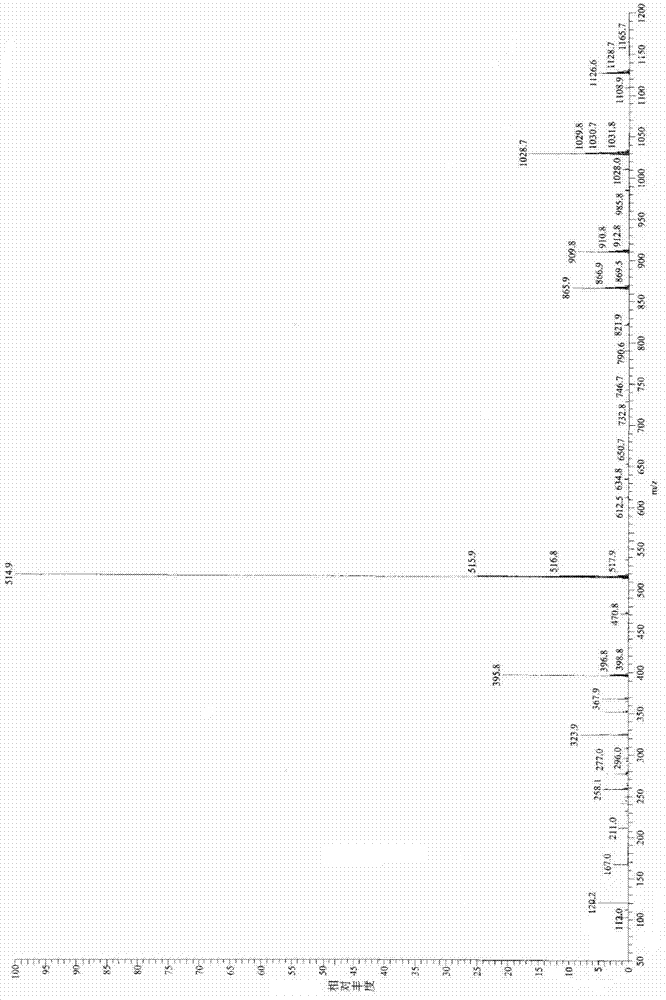
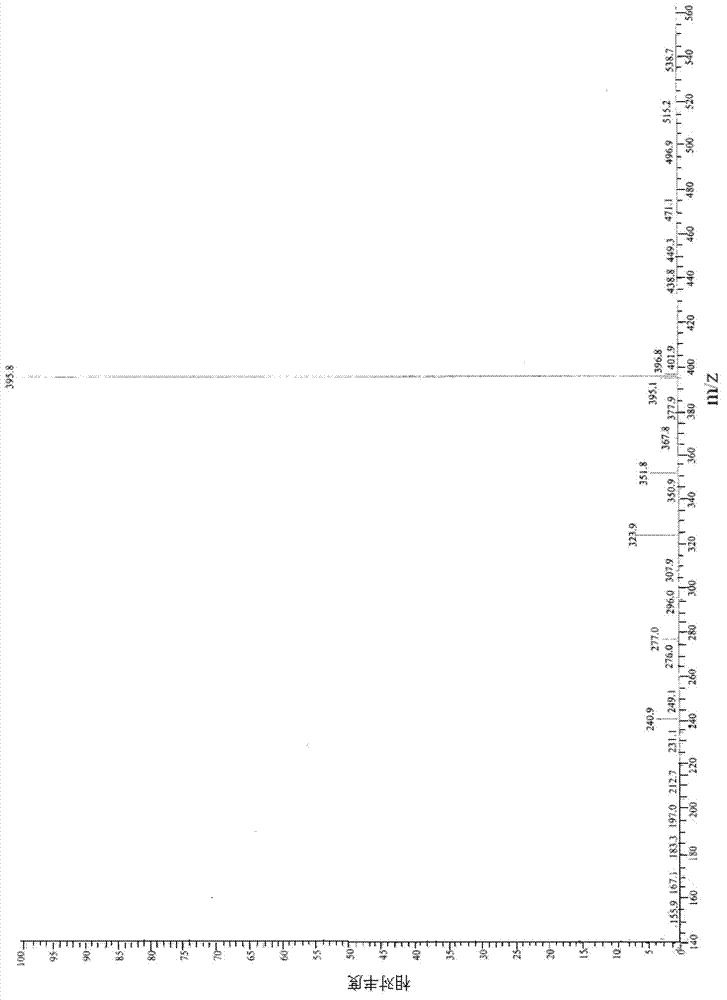
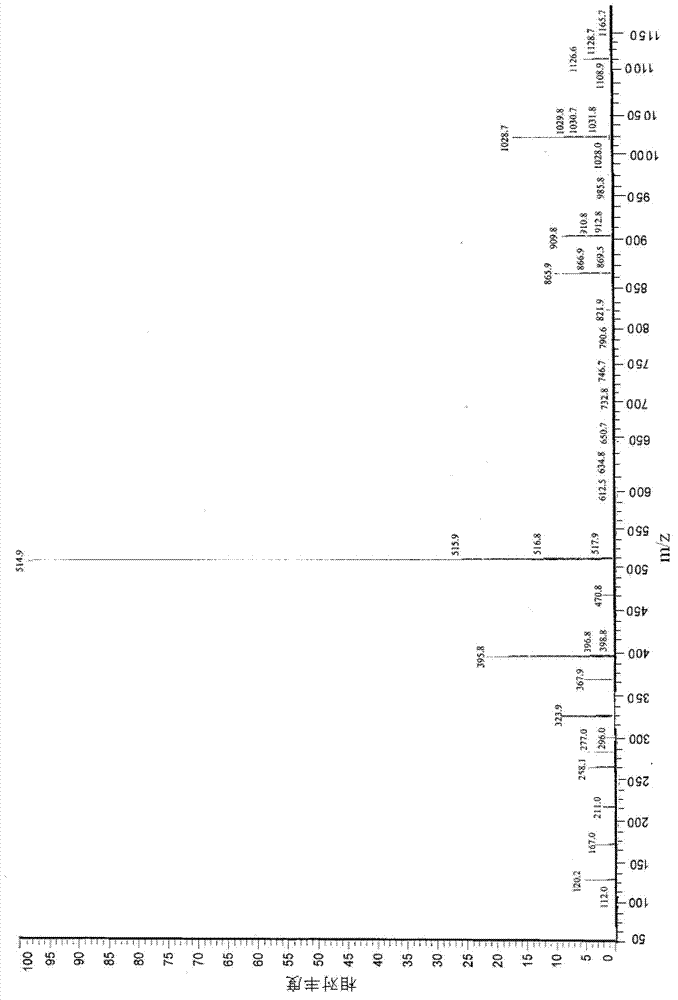
Preparation method for industrial amplified production of anagrelide hydrochloride active pharmaceutical ingredient
A technology for anagrelide hydrochloride and raw material medicines, which is applied in the field of preparation of raw material medicines, can solve the problems of long synthetic route of anagrelide hydrochloride, difficulty in product purification, and many impurities, and achieves less impurities, low cost per unit consumption, and Simple Effects of Mass Spectrometry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0057] Specific embodiment one: a kind of preparation method for industrial scale-up production of anagrelide hydrochloride crude drug of this embodiment is carried out according to the following steps:
[0058] 1) Preparation of 2,3-dichloro-6-nitrobenzonitrile
[0059] 2. Mix 2,3-dichlorobenzonitrile and dichloromethane uniformly at a mass volume ratio of 1g:8mL to obtain solution A; add mixed acid to solution A at a temperature of 10°C~15°C, and then ℃ ~ 25 ℃ temperature reaction, use TLC tracking during the reaction, when no 2,3-dichlorobenzonitrile is detected in the reaction system, the reaction is completed, and the reaction mixture is obtained; wherein, the mixed acid is obtained by mass The percentage content is 65%~70% HNO 3 solution and 98% H by mass 2 SO 4 The solution is mixed according to the volume ratio of 1:1;
[0060] 2. Mix the reaction mixture obtained in step 1 with water at 0°C~4°C in a ratio of 1:5 by volume, divide into two layers after stirring, co...
Embodiment approach 6
[0088] Specifically, Embodiment 6: The difference between this embodiment and one of Embodiments 1 to 5 is: N-(-2,3-dichloro-6-nitrophenyl) glycine ethyl ester hydrochloride described in step ten Mix evenly with concentrated hydrochloric acid at a mass volume ratio of 1g:810mL. One of other specific embodiments 1 to 5 is the same.
[0089] Specifically, embodiment seven: the difference between this embodiment and one of the specific embodiments one to six is that the mass volume ratio of the filter cake B described in step 12 to distilled water is 1g:5mL, anhydrous Na 2 SO 4 The mass volume ratio with ethyl acetate is 2g: 10mL. One of other specific embodiments 1 to 6 is the same.
[0090] Specifically Embodiment 8: The difference between this embodiment and one of Embodiments 1 to 7 is that the mass-to-volume ratio of the solid-phase substance and distilled water described in step 14 is 10 g: 2-5 mL. One of other specific embodiments 1 to 7 is the same.
[0091] Specif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com