Method for high selectivity recognition of acetate ions by applying perimidine onium anion receptor
A technology with high selectivity for acetate ions, which is applied in the field of recognizing acetate ions and using pyridinium anion receptors to recognize acetate ions with high selectivity, achieving the effect of simple operation, high selectivity recognition and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
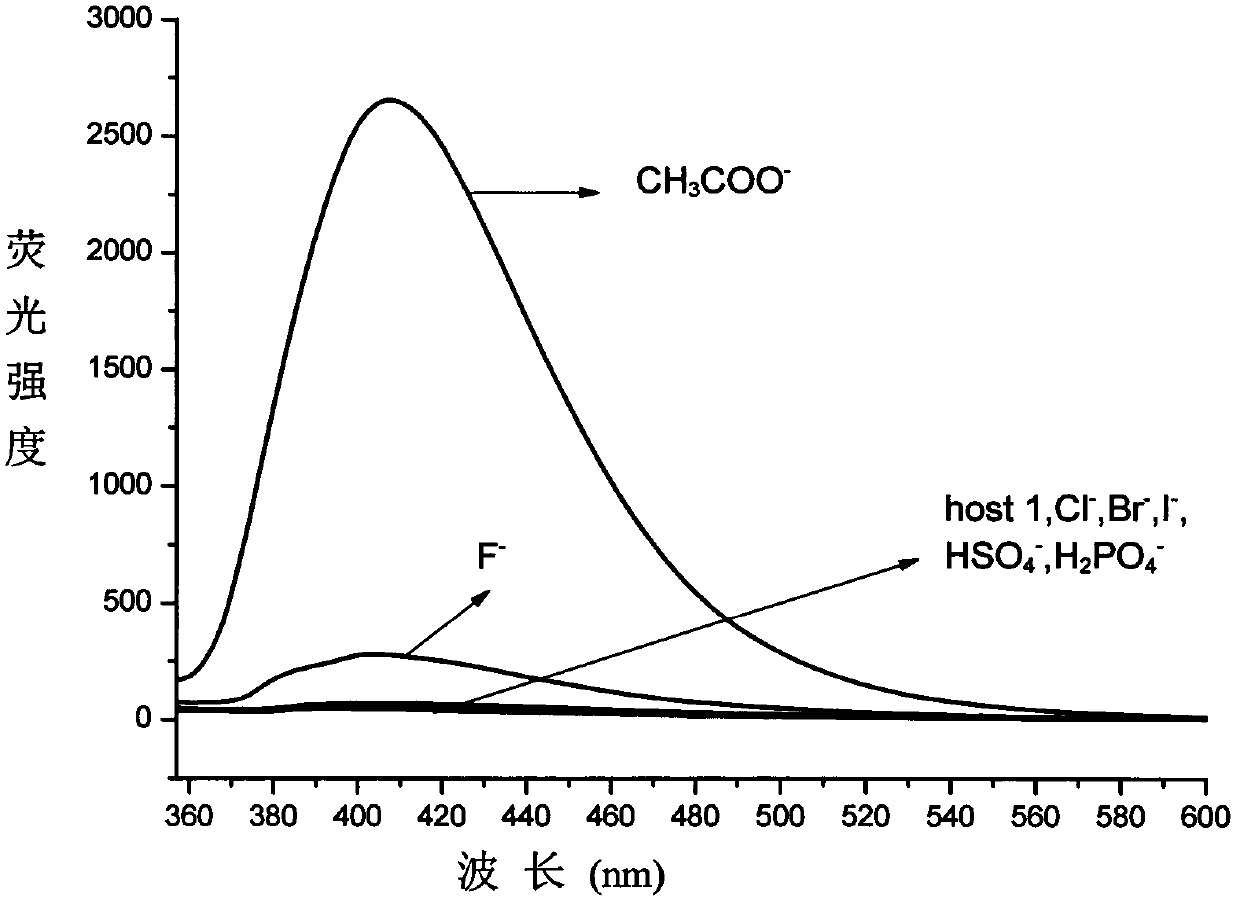
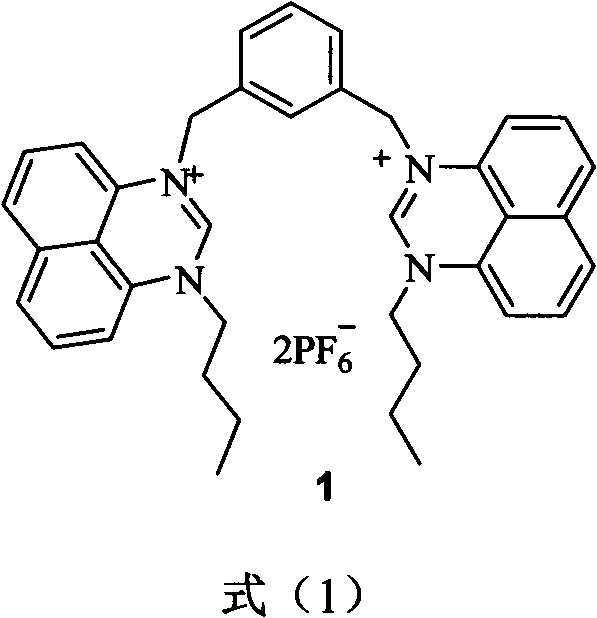
[0024] Pipette 1mL respectively Dimethylsulfoxide (DMSO) solution of pyridinium anion acceptor 1 (2.0×10 -6 mol / L) in a series of 10mL volumetric flasks, respectively add the sample to be tested: 0.5mL of tetrabutyl DMSO solution of ammonium salt (2.0×10 -5 mol / L), diluted to the mark with DMSO, at this time The pyridinium anion acceptor concentration was 2.0 x 10 -7 mol / L, the anion concentration is 1.0×10 -6 mol / L, that is, the concentration of each anion is 5 times the concentration of the acceptor, mixed well and left overnight, the concentration is 2.0×10 -7 The mol / L DMSO solution of pure acceptor was used as a control, and the fluorescence emission spectra of each solution were measured at 25°C. Comparing the changes in fluorescence intensity before and after adding various anion acceptors, the experimental results show that: after adding acetate ions, The fluorescence emission spectrum of the pyridinium anion acceptor at 402nm was significantly enhanced, and th...
Embodiment 2
[0026] Pipette 1mL respectively DMSO solution of pyridinium anion acceptor 1 (6.0×10 -6 mol / L) in a series of 10mL volumetric flasks, respectively add the sample to be tested: 0.5mL of tetrabutyl DMSO solution of ammonium salt (6.0×10 -5 mol / L), diluted to the mark with DMSO, at this time The pyridinium anion acceptor concentration was 6.0 x 10 -7 mol / L, the anion concentration is 3.0×10 -6 mol / L, that is, the concentration of each anion is 5 times the concentration of the acceptor, mixed well and left overnight, the concentration is 6.0×10 -7 The mol / L DMSO solution of pure acceptor was used as a control, and the fluorescence emission spectra of each solution were measured at 25°C. Comparing the changes in fluorescence intensity before and after adding various anions, the experimental results show that: after adding acetate ions, The fluorescence emission spectrum of the pyridinium anion acceptor at 402nm was significantly enhanced, and the fluorescence intensity inc...
Embodiment 3
[0028] Pipette 1mL respectively DMSO solution of pyridinium anion acceptor 1 (6.0×10 -5 mol / L) in a series of 10mL volumetric flasks, respectively add the sample to be tested: 0.5mL of tetrabutyl DMSO solution of ammonium salt (6.0×10 -4 mol / L), diluted to the mark with DMSO, at this time The pyridinium anion acceptor concentration was 6.0 x 10 -6 mol / L, the anion concentration is 3.0×10 -5 mol / L, that is, the concentration of each anion is 5 times the concentration of the acceptor, mixed well and left overnight, the concentration is 6.0×10 -6 The mol / L DMSO solution of pure acceptor was used as a control, and the fluorescence emission spectra of each solution were measured at 25°C. Comparing the changes in fluorescence intensity before and after adding various anions, the experimental results show that, if figure 1 As shown, after adding acetate ion, The fluorescence emission spectrum of the pyridinium anion acceptor at 402nm was significantly enhanced, and the fluo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com