Method for inducing migration of adult stem cells derived from adipose tissue
A technology of adult stem cells and adipose stem cells, applied in animal cells, vertebrate cells, artificial cell constructs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1: Isolation of human adipose tissue-derived mesenchymal stem cells
[0095] Human adipose tissue was isolated from abdominal fat by liposuction and washed with PBS. The isolated tissue was finely dissected and then digested with DMEM medium supplemented with collagenase type 1 (1 mg / ml) at 37°C for 2 hours. After washing with PBS, the digested tissue was centrifuged at 1000 rpm for 5 minutes. The supernatant was aspirated off, and the pellet remaining at the bottom was washed with PBS, and then centrifuged at 1000 rpm for 5 minutes. Remaining cells were filtered through a 100-μm mesh filter to remove debris, cells were washed with PBS and then cultured overnight in DMEM (10% FBS, 2 mM NAC, 0.2 mM ascorbic acid).
[0096] Subsequently, non-attached cells were removed by washing with PBS, and the remaining cells were subcultured, while every two days in Keratinocyte-SFM medium (containing 5% FBS, 2mM NAC, 0.2mM ascorbic acid, 0.09mM calcium, 5ng / ml rEGF, 5 μ...
Embodiment 2
[0097] Example 2: Inducing Migration of Adipose Stem Cells
[0098] 2-1: Induction of cell migration by chemokines or growth factors shown in Table 2
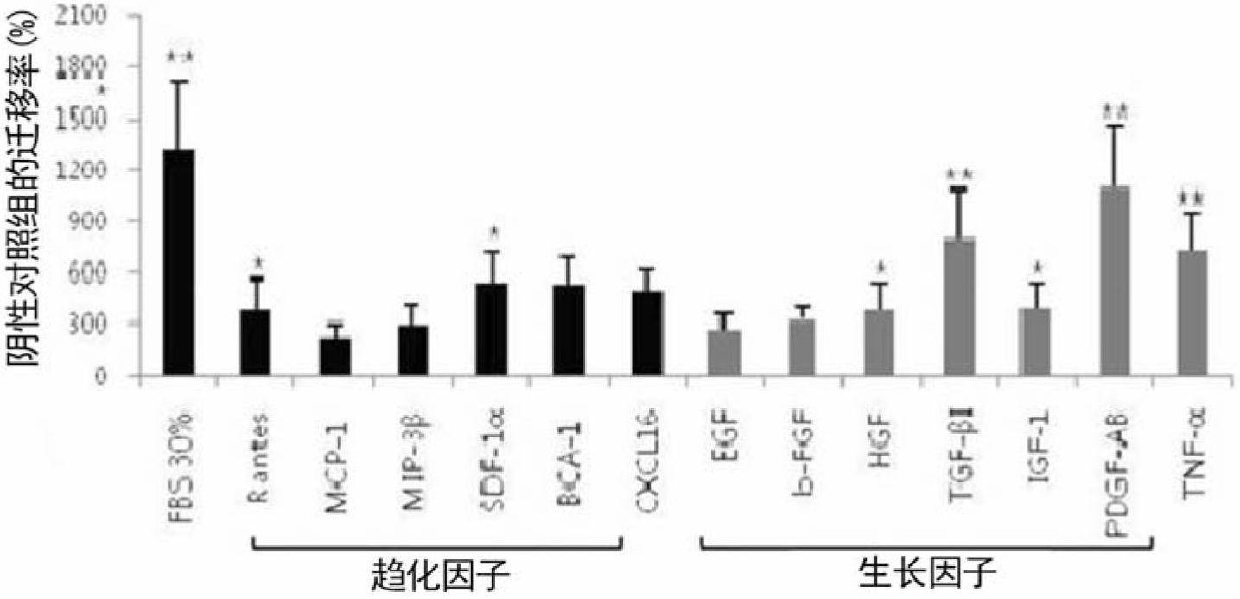
[0099] The adipose tissue-derived pluripotent mesenchymal stem cells isolated in Example 1 were mixed with 2×10 4 Cells were seeded at a concentration of 200 μl into each well and induced to migrate by the chemokines or growth factors shown in Table 2 below. 30% FBS was used as the positive control group.
[0100] Table 2
[0101] FBS 30%
BCA-1
TGF-β1
Rantes
CXCL16
IGF-1
MCP-1
EGF
PDGF-AB
MIP-3β
b-FGF
TNF-α
SDF-1α
HGF
[0102] figure 1Results for inducing cell migration are shown. Cells induced to migrate by culture medium were used as negative control group, and cells induced to migrate by 30% FBS were used as positive control group. The result of inducing cell migration was calculated as the relative percentage of 100% negative control...
Embodiment 3
[0108] Example 3: Migration of pretreated adipose stem cells with chemokines or growth factors
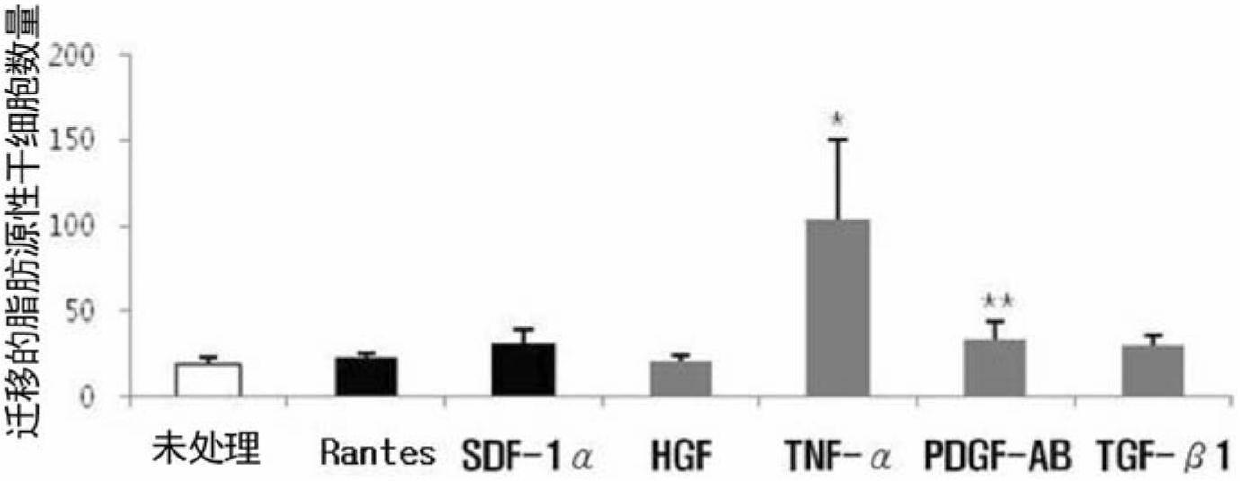
[0109] The adipose tissue-derived multipotent mesenchymal stem cells isolated in Example 1 were pretreated with the chemokines or growth factors shown in Table 3 below for 24 hours. The pretreated cells were divided into 2×10 4 The concentration of cells / 200 μl was seeded into each well, and the migration was induced by 10% FBS to observe the difference from the induction of cell migration by 30% FBS. At the same time, untreated adipose-derived multipotent mesenchymal stem cells (untreated cells) were used as a negative control group.
[0110] table 3
[0111] Rantes
[0112] image 3 Results for inducing cell migration are shown. From image 3 The indicated numbers of migrated cells can be seen in these adipose tissue-derived mesenchymal stem cells (AdMSCs) pre-treated with different chemokines or growth factors, which were actively induced by 10% FBS and induced ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com