Piperazines as antimalarial agents
A technology of piperazine and compounds, applied in the field of preparation of these compounds, can solve problems such as the rapid spread of drug resistance and threats to malaria endemic areas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0136] Compounds of formula I according to the invention may be prepared according to the procedures described herein, in particular as described in the Examples section.
[0137] In general, all chemical transformations can be carried out according to well-known standard methods as described in literature sources or as described in the following procedures.
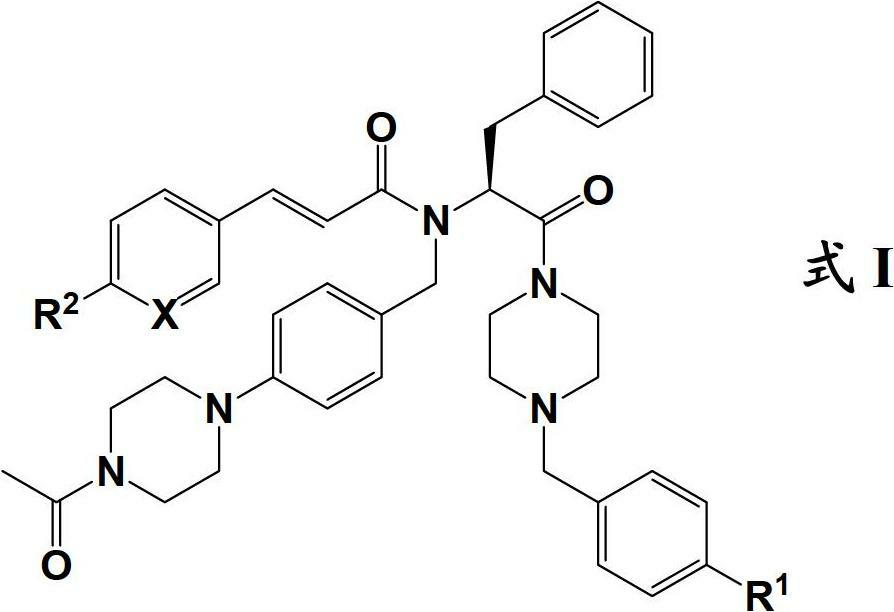
[0138] except R 1 Yes - NCH 3 (CH 2 CH 2 OH) The preparation method of the compound of formula I except the compound:
[0139]
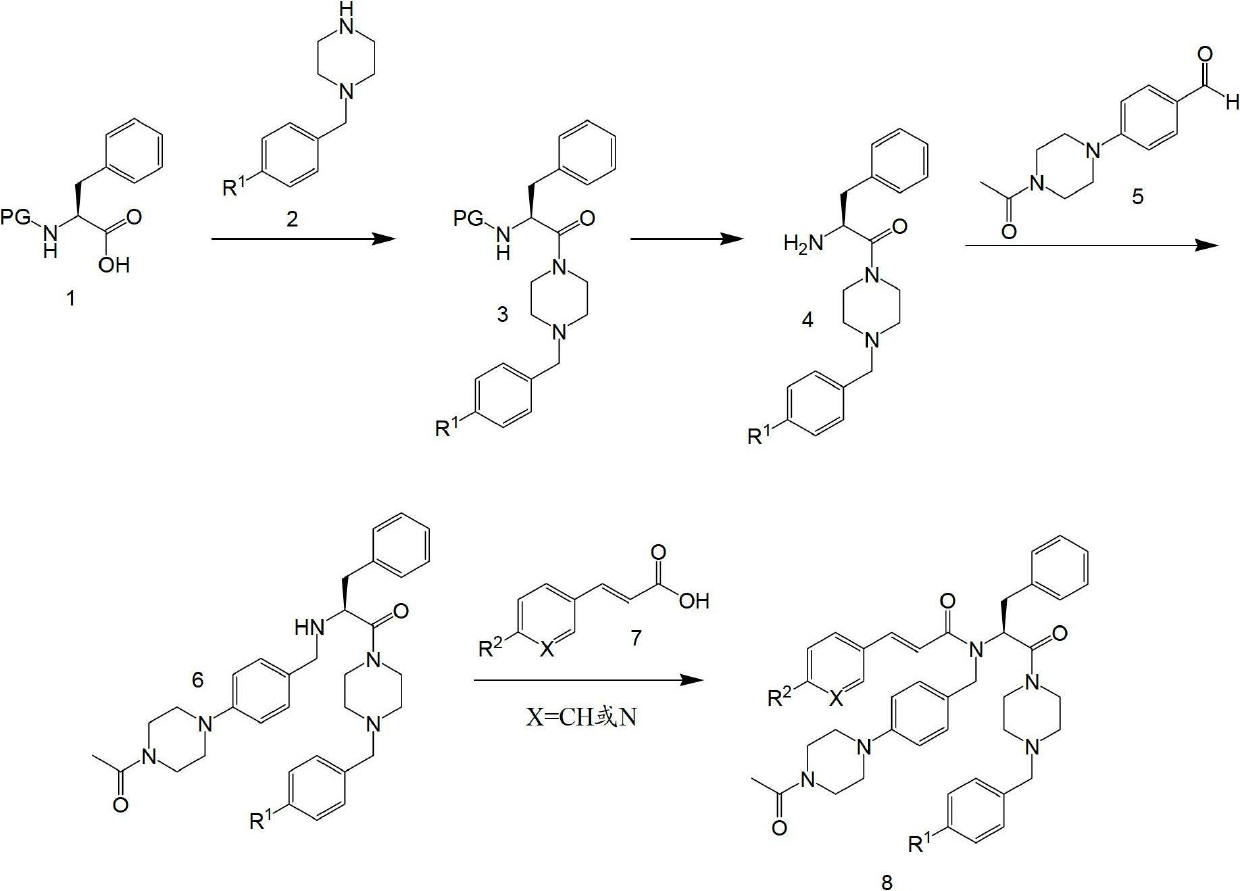
[0140] plan 1
[0141] Boc-Phe- OH 1 is coupled with benzylpiperazine derivative 2 to obtain intermediate 3. Alternatively, Cbz-Phe-OH can also be used in the initial peptide coupling step to obtain 3. Boc-deprotection is usually achieved by reacting 3 with HCl in 4N dioxane (using DCM as solvent), while Cbz-deprotection is achieved by hydrogenation in MeOH with a Pd / C catalyst to obtain the amine intermediate Body 4. Reductive amination of free amine 4 with aldehyde 5 in MeOH at re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com