Combination
A technology of compounds and solvates, applied in the field of treating cancer in mammals, can solve the problem of low frequency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0102] Preparation of MEK inhibitors
[0103] MEK inhibitors suitable for use in the combinations according to the invention, in particular
[0104] N-{3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-6,8-dimethyl-2,4,7-trioxo-3,4 ,6,7-Tetrahydropyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl}acetamide dimethyl sulfoxide
[0105]
[0106] It can be prepared according to International Patent Publication WO2005 / 121142.
[0107] PI3K inhibitors suitable for use in the combinations of the invention, in particular
[0108] 2,4-Difluoro-N-{2-(methoxy)-5-[4-(4-pyridazinyl)-6-quinolinyl]-3-pyridyl}benzenesulfonamide
[0109]
[0110] It can be prepared according to International Patent Publication No. WO08 / 144463 (Example 345).
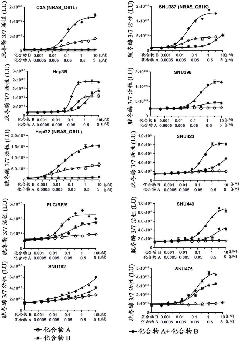
[0111] Compound A described in the experimental part refers to N-{3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-6,8-dimethyl-2, dimethylsulfoxide solvate of 4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl}acetamide.
[0112] In v...
Embodiment 1
[0222] Example 1 - Capsule Composition
[0223] Oral dosage forms for administering the combinations of the invention are prepared by filling standard two-part hard gelatin capsules with the ingredients in the proportions shown in Table I below.
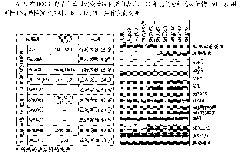
[0224] Table I
[0225]
[0226]
Embodiment 2
[0227] Example 2 - Capsule Composition
[0228] Oral dosage forms for administering one of the compounds of the invention are prepared by filling standard two-part hard gelatin capsules with the ingredients in the proportions shown in Table II below.
[0229] Table II
[0230]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com