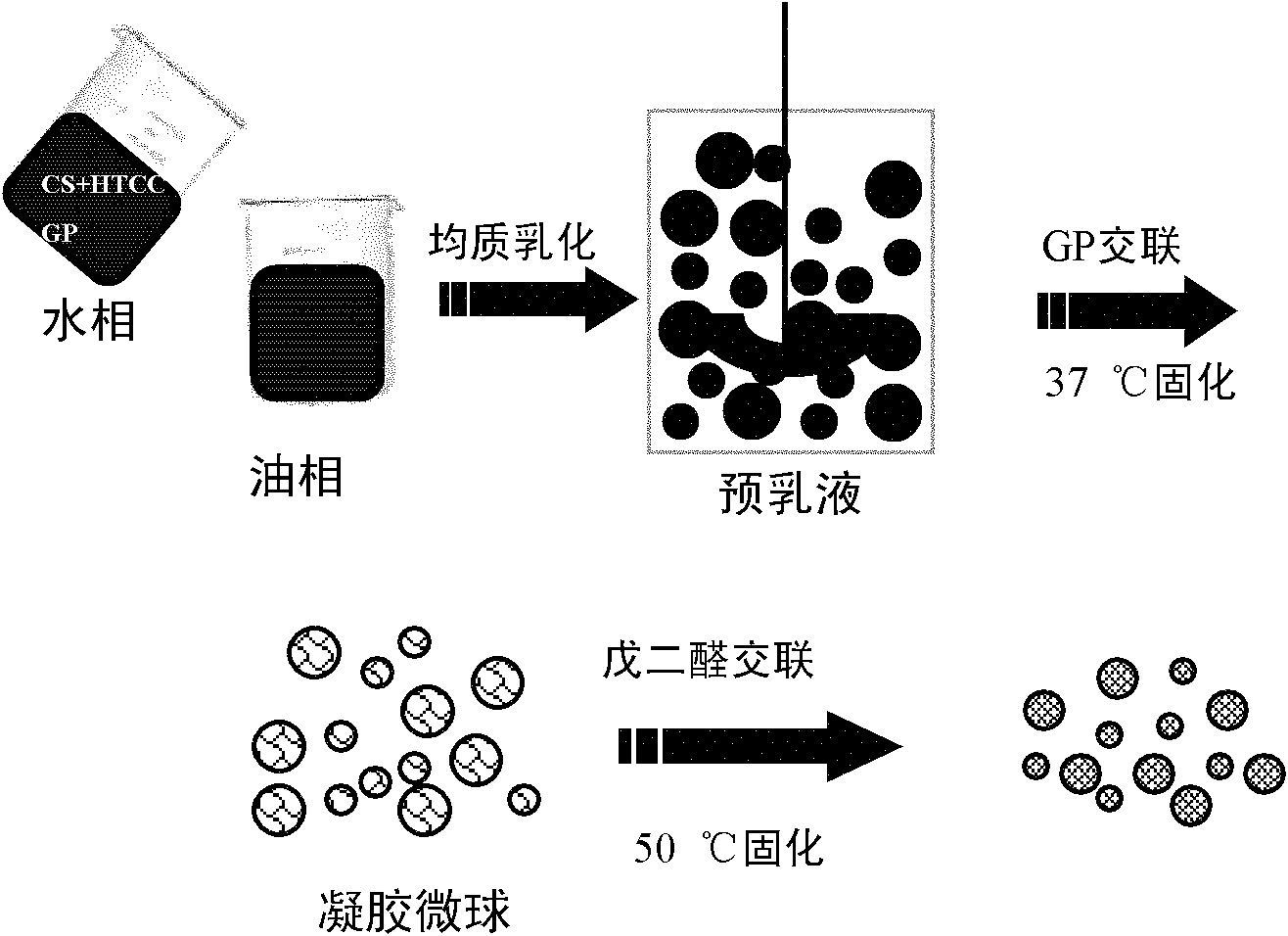
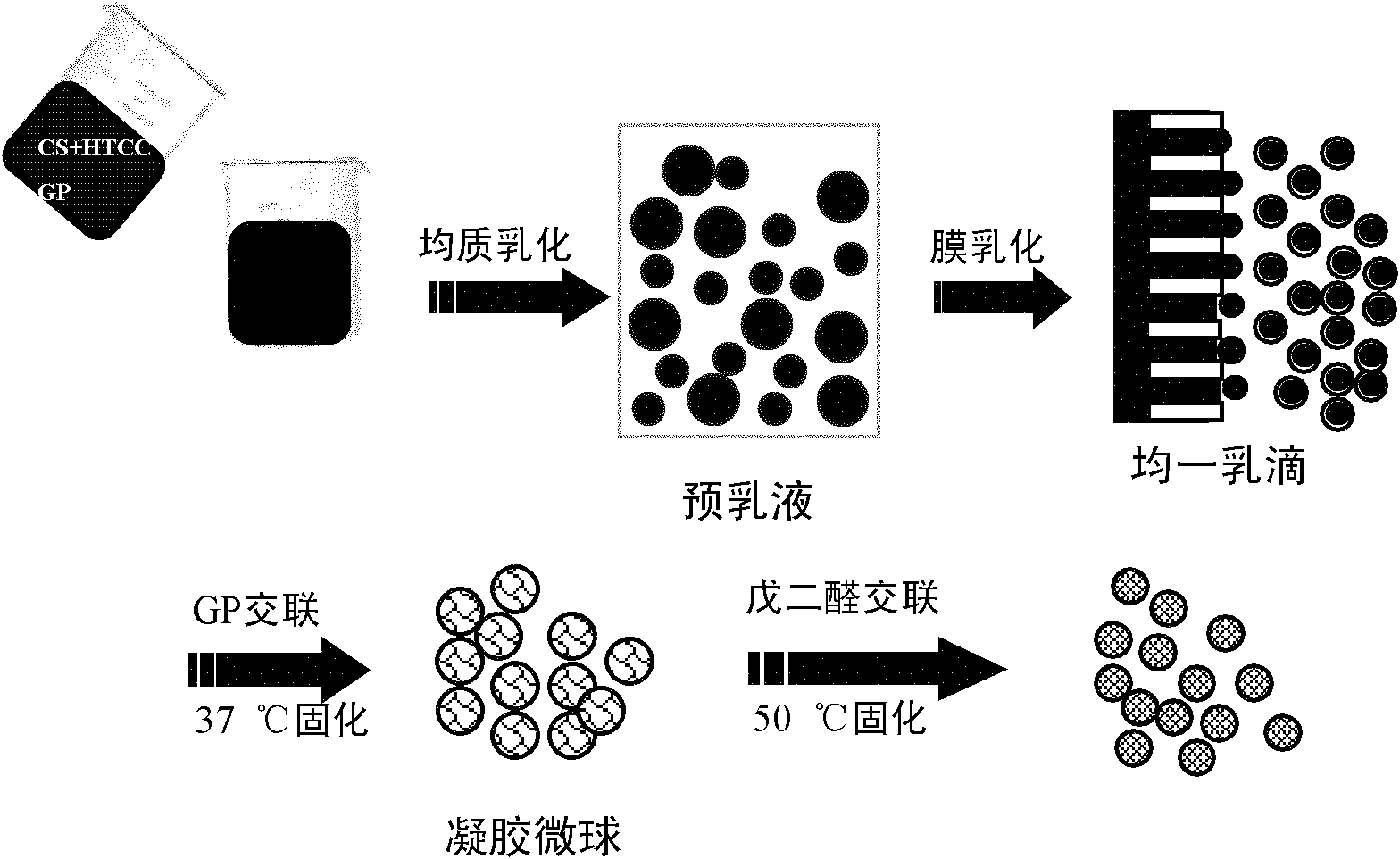
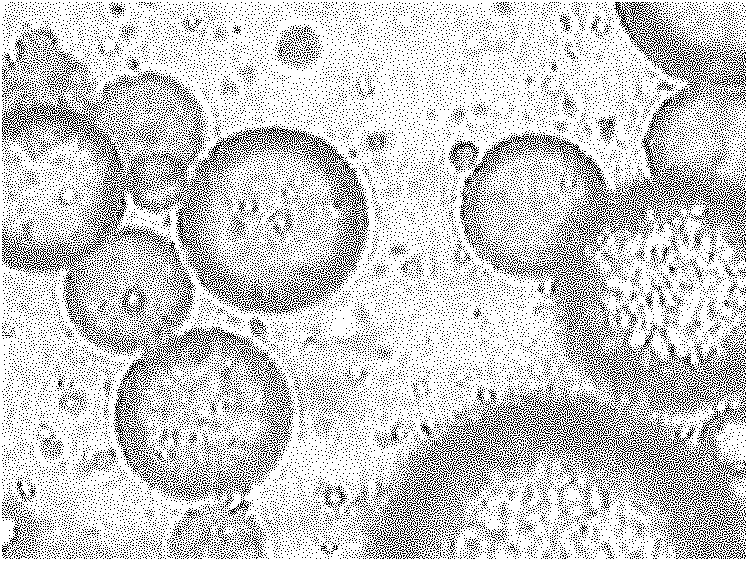Gastric targeted drug carrier and preparation method thereof
The technology of a drug and a carrier is applied in the field of gastric targeted drug carrier and its preparation, which can solve the problems of losing the targeting effect, unable to satisfy the gastric targeted drug delivery carrier, unable to achieve the drug sustained-release performance, etc., and achieves stability Autofluorescence, good biocompatibility, the effect of improving the embedding rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Accurately weigh chitosan (molecular weight 50,000), chitosan quaternary ammonium salt (quaternary ammonium substitution degree 50%, molecular weight 50,000), sodium glycerophosphate and dissolve it in 1% acetic acid aqueous solution, and make it fully dissolve under magnetic stirring to obtain The acetic acid aqueous solution of chitosan, chitosan quaternary ammonium salt, sodium glycerophosphate, its total concentration is 2.5wt%, wherein the concentration of chitosan, chitosan quaternary ammonium salt is respectively 0.5wt%, the concentration of sodium glycerophosphate The mass ratio of chitosan and chitosan quaternary ammonium salt is 1:1, and the mass ratio of chitosan and chitosan quaternary ammonium salt to sodium glycerophosphate is 2:3. The solution was centrifuged at 8000 rpm to remove insoluble impurities, and the supernatant was retained as the aqueous phase for subsequent use. Take 400 μL of 10 mg / mL rapamycin solution O 1 (rapamycin is dissolved in chloro...
Embodiment 2
[0059] Accurately weigh chitosan (molecular weight 50,000), chitosan quaternary ammonium salt (quaternary ammonium substitution degree 50%, molecular weight 50,000), sodium glycerophosphate and dissolve it in 1% acetic acid aqueous solution, and make it fully dissolve under magnetic stirring to obtain The acetic acid aqueous solution of chitosan, chitosan quaternary ammonium salt, sodium glycerophosphate, its total concentration is 2.5wt%, wherein the concentration of chitosan, chitosan quaternary ammonium salt is respectively 0.5wt%, the concentration of sodium glycerophosphate The mass ratio of chitosan and chitosan quaternary ammonium salt is 1:1, and the mass ratio of chitosan and chitosan quaternary ammonium salt to sodium glycerophosphate is 2:3. This solution was centrifuged at 8000rpm to remove insoluble impurities, then added 20 wt% gentamicin sulfate (stomach medicine) of the total dry weight of chitosan and chitosan quaternary ammonium salt and fully dissolved for fu...
Embodiment 3
[0061] Accurately weigh chitosan (molecular weight 50,000), chitosan quaternary ammonium salt (quaternary ammonium substitution degree 70%, molecular weight 100,000), sodium glycerophosphate and dissolve it in 1% acetic acid aqueous solution, and make it fully dissolve under mechanical stirring to obtain The acetic acid aqueous solution of chitosan, chitosan quaternary ammonium salt, sodium glycerophosphate, its total concentration is 2.5wt%, wherein the concentration of chitosan, chitosan quaternary ammonium salt is respectively 0.5wt%, the concentration of sodium glycerophosphate The mass ratio of chitosan and chitosan quaternary ammonium salt is 1:1, and the mass ratio of chitosan and chitosan quaternary ammonium salt to sodium glycerophosphate is 2:3. The solution was centrifuged at 8000 rpm to remove insoluble impurities, and the supernatant was retained as the aqueous phase for subsequent use. Take 400 μL of 10 mg / mL rapamycin solution O 1 (rapamycin is dissolved in chl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com