2,6-bis-(1,1'-naphthalene amino azo)benzo(1,2-d;4,5-d') dithiazole, and preparation method and application thereof
A naphthylaminoazo, 2-d technology, applied in the field of bistriazene compounds, can solve the problems of unsatisfactory reagent sensitivity and the like, and achieve the effects of high sensitivity, good selectivity and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
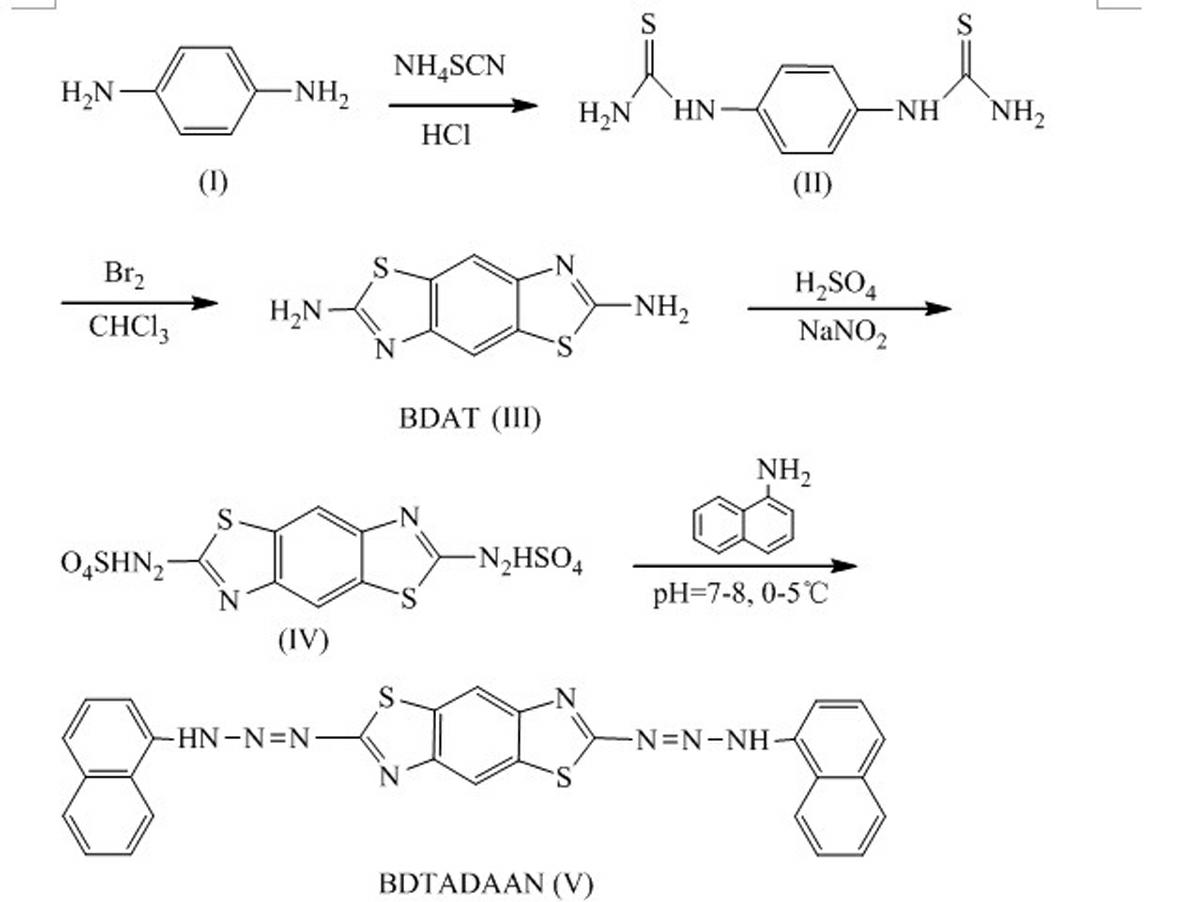
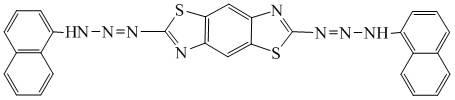
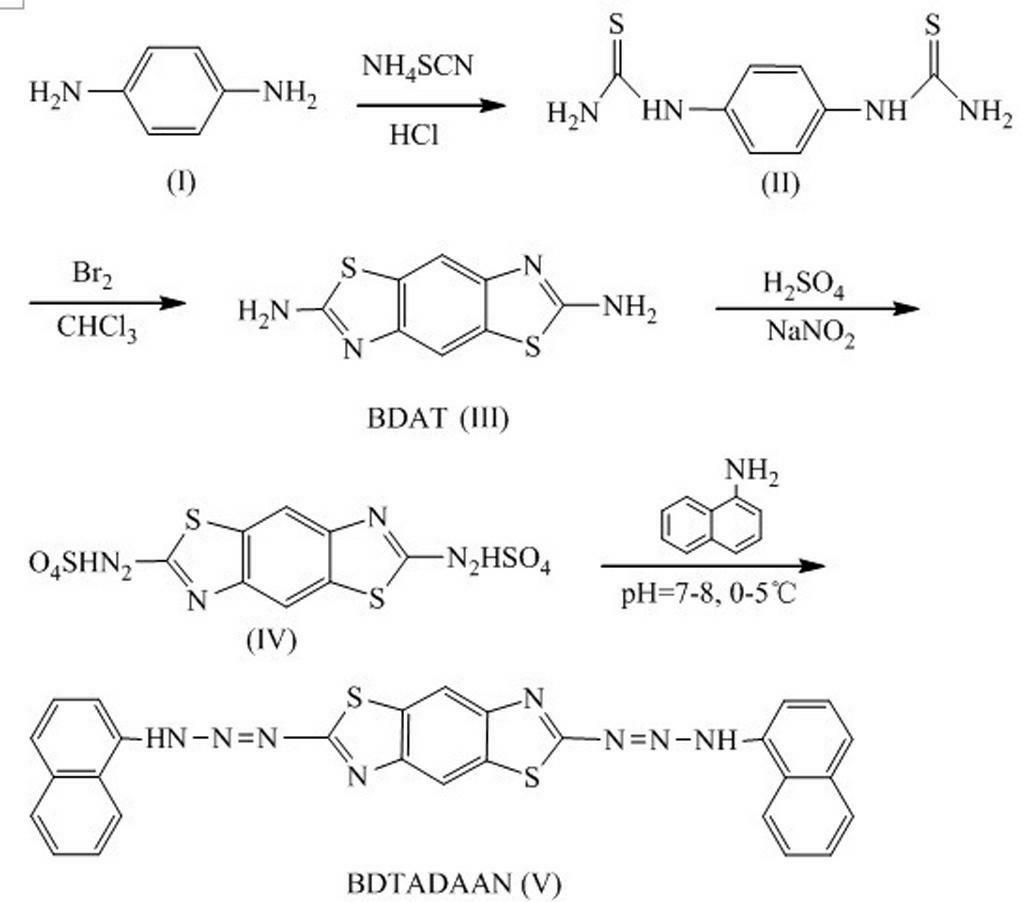
[0033] The present invention is achieved by the following technical scheme, a 2,6-bis-(1,1′-naphthylaminoazo)benzo(1,2-d;4,5-dˊ)bithiazole (BDTADAAN for short) )
[0034] Its molecular structural formula is:
[0035]
[0036] 2,6-bis-(1,1′-naphthylaminoazo)benzo(1,2-d; 4,5-dˊ)bithiazole preparation method, such as figure 1 The illustrated process includes the following steps:
[0037] (1) Preparation of p-phenylenedithiourea Add 8.5 g (0.079 mol) p-phenylenediamine in a 110 mL flask, remove O 2 water, 15.4 mL of concentrated hydrochloric acid and 0.6 g of activated carbon. This mixture was heated to 50 °C. Transfer it to another 110 mL flask by filtering, add 24.2 g (0.318 mol) ammonium thiocyanate, stir the mixture at a temperature of 90-100 °C for 20-24 h, and precipitate out after 2 h of reaction The yellow granular product was collected by filtration after cooling, then washed with 40 mL of hot water, and dried under reduced pressure at 100 °C to obtain 14.0 g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com