Bis-Schiff bases synthesized by condensing indole-3-carboxaldehyde and benzidine and preparation method thereof
A technology of formaldehyde diphenylenediamine and bis-Schiff base, which is applied in the fields of chemical instruments and methods, luminescent materials, organic chemistry, etc., and can solve problems such as no related reports on synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Weigh 1.84g (0.01mol) of benzidinediamine and 2.89g (0.02mol) of indole-3-carboxaldehyde, dissolve in 50ml of acetone, and add to a 100ml four-necked bottle equipped with a thermometer and a stirring device . Stirring was started, the reaction temperature was controlled at 25°C, and the constant temperature was reacted for 3 hours. The solvent was distilled off under reduced pressure to obtain a yellow solid powder, which was recrystallized with ethanol and dried in vacuum at 50°C for 6 hours to obtain the target product: indole-3-formaldehyde condensate Biphenyldiamine bis-Schiff base.
[0033] Elemental Analysis: C 30 h 22 N 4 : %C: 82.17 (82.15); %H: 5.06 (5.00); %N: 12.78 (12.85) (measured values in brackets).
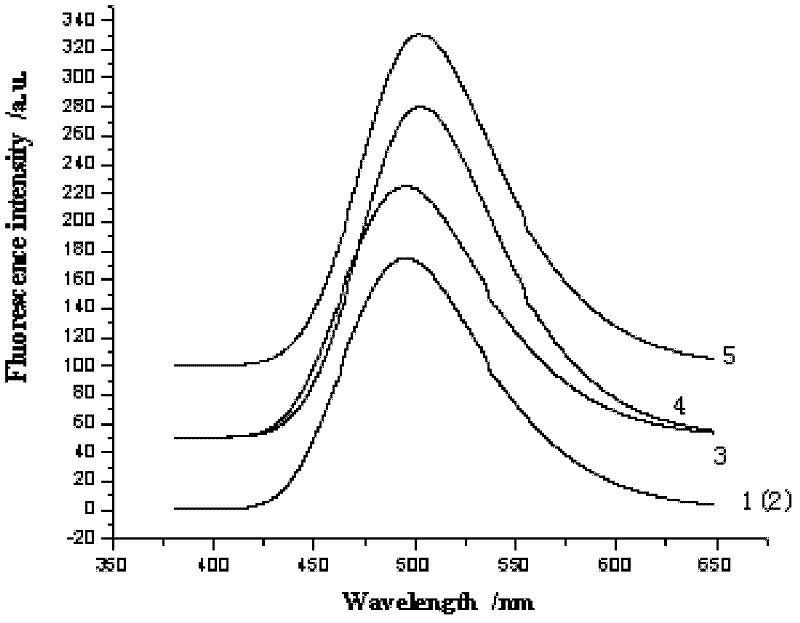
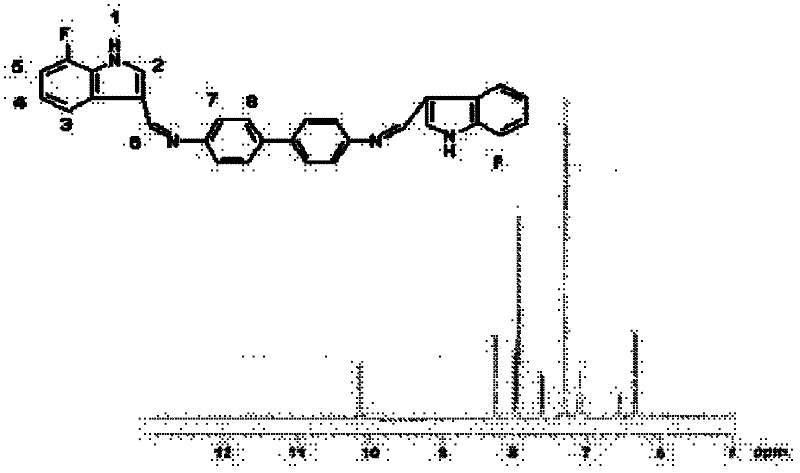
[0034] NMR analysis (NMR spectrum see figure 2 ):
[0035] Table 1 was obtained by analyzing the structural formula and proton nuclear magnetic resonance spectrum of compound 1. Compound 1 has nine kinds of hydrogen in total, and the peak that appea...
Embodiment 2
[0040] Weigh 1.84g (0.01mol) of benzidinediamine, weigh 2.89g (0.02mol) of indole-3-carboxaldehyde, dissolve in 50ml of anhydrous methanol, and add it to a 100ml four-port tube equipped with a thermometer and a stirring device. in the bottle. Stirring was started, the reaction temperature was controlled at 35°C, and the constant temperature reaction was carried out for 12 hours. The solvent was distilled off under reduced pressure to obtain a white solid powder, which was recrystallized with ethanol and dried in vacuum at 50°C for 6 hours to obtain the target product: indole-3-formaldehyde acetal Biphenyldiamine bis-Schiff base.
[0041] Elemental Analysis: C 30 h 22 N 4 : %C: 82.17 (83.15); %H: 5.06 (5.10); %N: 12.78 (11.75) (measured values in brackets).
[0042] NMR analysis (NMR spectrum see figure 2 ): see compound 1 for the analysis results.
Embodiment 3
[0044] Weigh 1.84g (0.01mol) of benzidinediamine, weigh 6.75g (0.05mol) of 7-fluoroindole-3-carboxaldehyde, dissolve in 50ml of methanol, and add to 100ml of tetrachloride equipped with a thermometer and a stirring device. in the bottle. Stirring was started, the reaction temperature was controlled at 35°C, and the constant temperature reaction was carried out for 8 hours. The solvent was distilled off under reduced pressure to obtain a yellow solid powder, which was recrystallized with ethanol and dried in vacuum at 50°C for 6 hours to obtain the target product: 7-fluoroindole-3 - Formaldehyde biphenylenediamine bis-Schiff base.
[0045] Elemental Analysis: C 30 h 20 N 4 f 2 : %C: 75.94 (75.84); %H: 4.25 (4.29); %N: 11.81 (11.71); %F: 8.01 (8.16) (measured values in brackets).
[0046] NMR analysis (NMR spectrum see image 3 ):
[0047]Table 2 was obtained by analyzing the structural formula and proton nuclear magnetic resonance spectrum of compound 2. Compound 2 ha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com