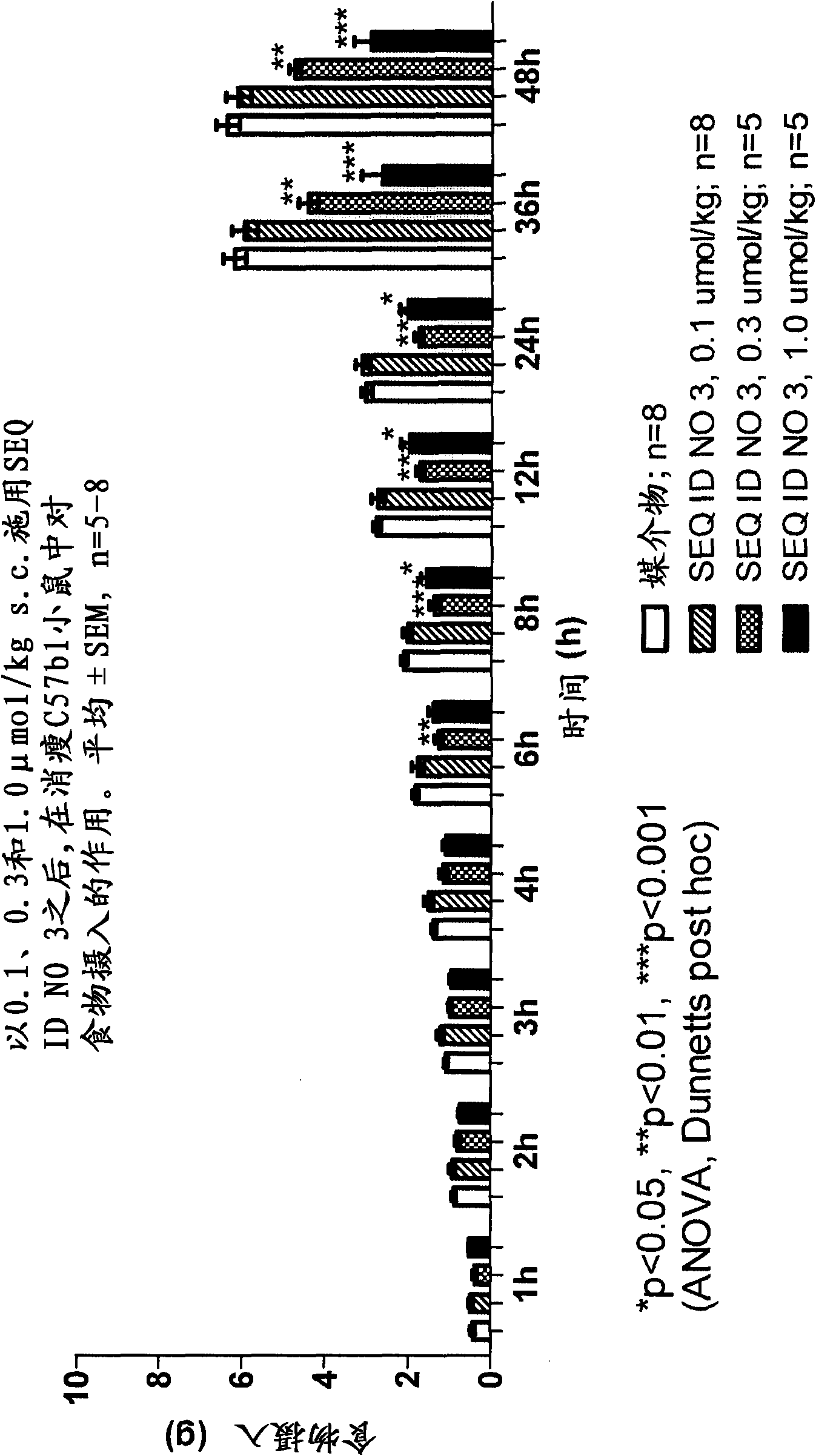
Long-acting Y2 receptor agonists
A technology of derivatives and analogues, applied in the field of therapeutic peptides, can solve problems such as inconvenience to patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
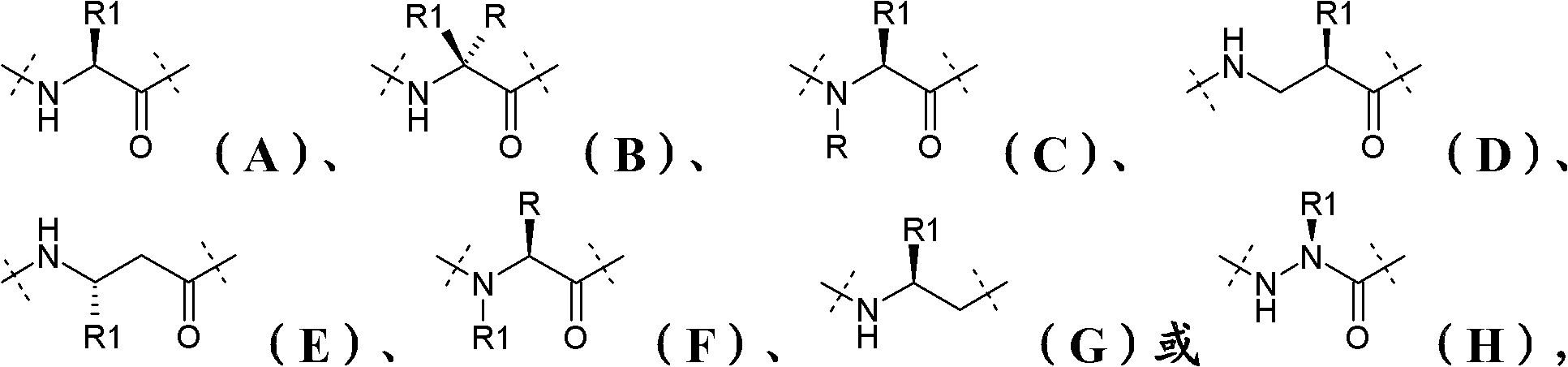
[0279] analog
[0280] 1. Compared with human PYY(1-36) or human PYY(3-36), there is a PYY analog or derivative thereof with improved stability for C-terminal proteolytic cleavage, which decomposes to reduce said analog or Derivative functionality.
[0281] 2. The PYY analog or derivative thereof according to any one of the preceding embodiments, wherein said PYY analog is not contained in PYY(1-36), PYY(2-36), PYY(3-36), PYY (4-36) or [N-methylTyr36] or [D-Ala3] in any of PYY(5-36).
[0282] 3. The PYY analogue or derivative thereof according to any one of the preceding embodiments, wherein said functionality is determined by assay (IV), in vitro half-life in plasma, as described herein.
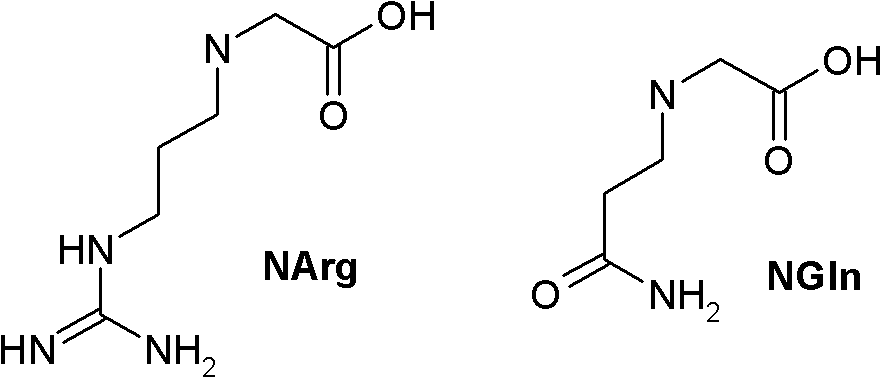
[0283] 4. The PYY analogue or derivative thereof according to any one of the preceding embodiments, wherein said analogue or derivative comprises the amino acid sequence of formula (I):
[0284] Xaa 1 -Xaa 2 -Xaa 3 -Xaa 4 -Xaa 5 -Xaa 6 -Xaa 7 -Xaa 8 -Xaa 9 -Xaa 10 -Xaa 11 -Xaa...
Embodiment 1
[0440] Synthesis of resin-bound peptides, SPPS method:The protected peptidyl resin was synthesized according to the Fmoc strategy on a Prelude Solid Phase Peptide Synthesizer from Protein Technologies on a 0.25 mmol scale using DIC and HOAt mediated coupling in NMP. The starting resin for the synthesis of peptide amides is Rink-Amide resin. The protected amino acid derivatives used are standard Fmoc-amino acids (supplied eg by Anaspec, Bachem, Iris Biotech, Watanabe or Novabiochem). In some cases, Fmoc-amino acids are custom synthesized using methods known in the art. The epsilon amino group of the lysine to be acylated was protected with Mtt. In some cases, peptide synthesis can be improved by using dipeptides such as pseudoproline from Novabiochem, Fmoc-Ser(tbu)-ψSer(Me,Me)-OH, see e.g. from Novabiochem 2002 / 2003 or A more recent version of the catalog, or W.R. Sampson (1999), J.Pep.Sci.5, 403.
[0441] Procedure for cleavage of peptides from resin: After synthesis, t...
Embodiment 2
[0492] A comparison of the in vitro half-lives determined for PYY(3-36) analogues in minipig plasma (assay (IV)) and Y2, Y1 and Y5 receptor potency (assays (I), (II) and (III) respectively) shows that in Table 2. This experiment shows that some modifications introduced into PYY prolong the half-life of PYY in vitro.
[0493] Table 2. In Vitro Half-Life of PYY(3-36) Compounds in Minipig Plasma and Potency at Y2, Y1 and Y5 Receptors (Y2R, Y1R and Y5R are Y2, Y1 and Y5 receptors, respectively).
[0494]
[0495]
[0496] n / a: not analyzed
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com