Enhancer of activity of nucleic acid analogue
A nucleic acid analog, active technology, applied in the field of drugs for viral hepatitis, prevention or treatment of viral hepatitis, to achieve the effects of enhancing activity, improving poor prognosis, and improving liver reserve function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
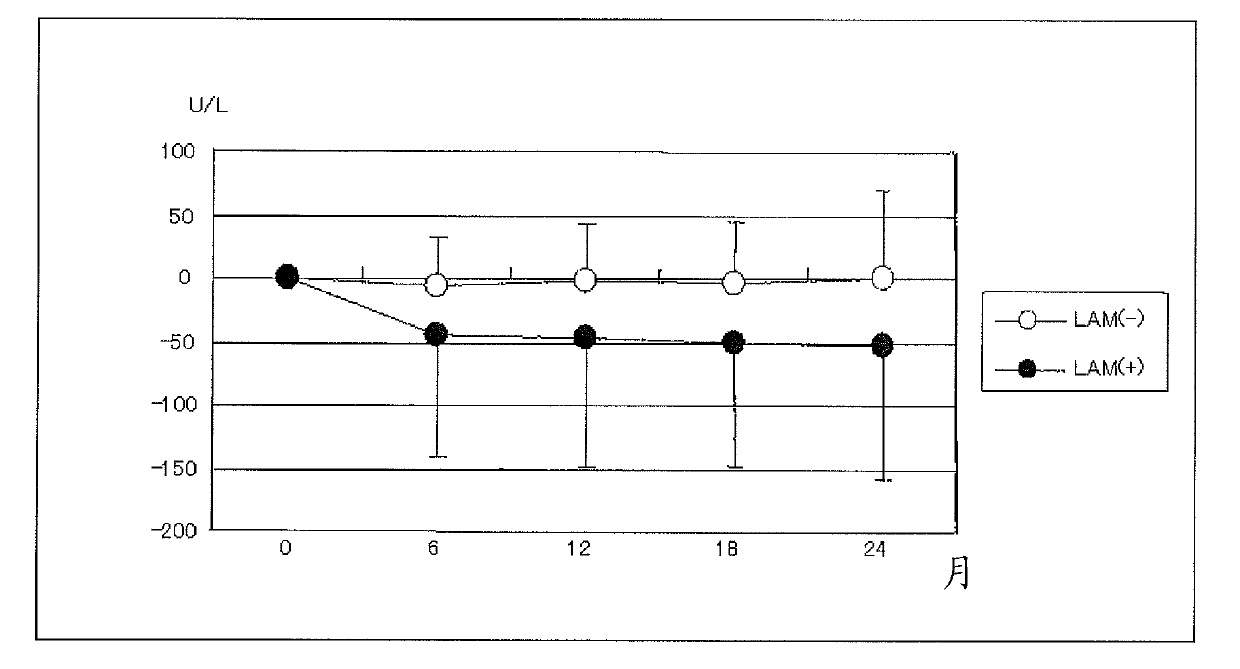
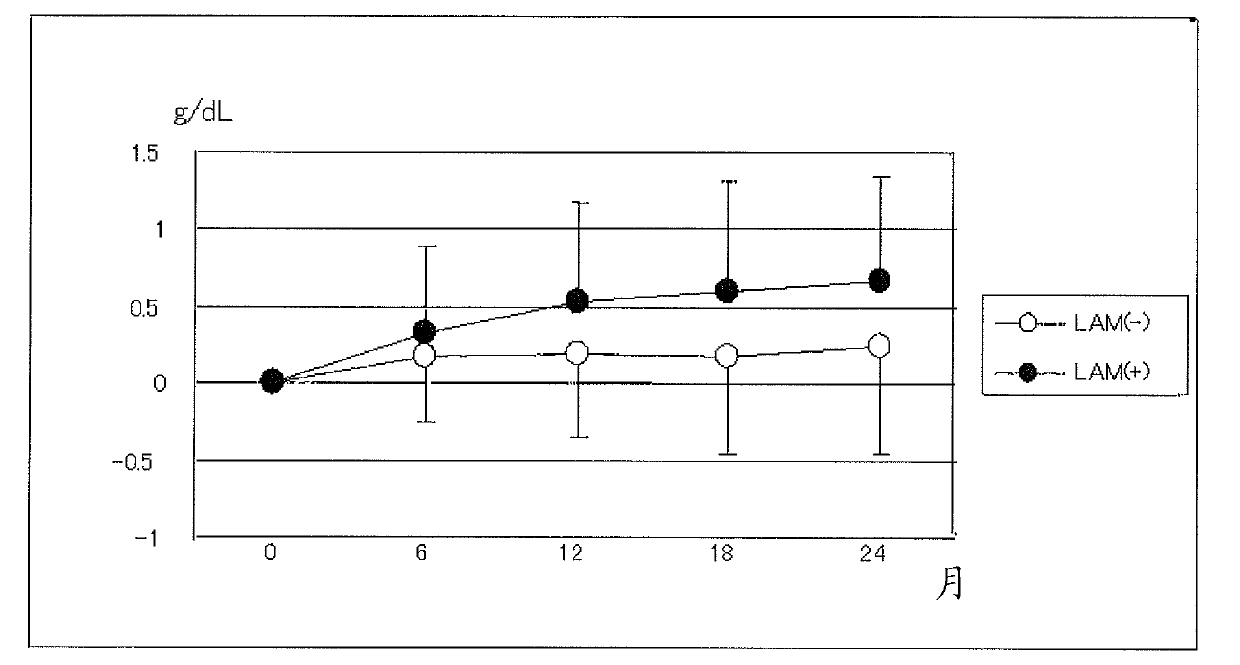
[0114] Embodiment 1: the joint application effect (1) of nucleic acid analog preparation and activity enhancer of the present invention
[0115] (method)
[0116] Oral administration of isoleucine, leucine, valine after meals in hepatitis B virus-positive patients who are decompensated cirrhotic patients who exhibit hypoalbuminemia despite adequate food intake Branched-chain amino acid preparation リーバクト (registered trademark) granules (Ajinomoto Co., Ltd.) with a weight ratio of 1:2:1.2 (isoleucine: 0.952 g, leucine: 1.904 g, valine: 1.144 g) , 3 times a day, 1 packet each time, and measure the changes of serum albumin value, AST and ALT value every day. The results were divided into 2 groups and analyzed according to the prescription history of the antiviral chemotherapeutic agent lamivudine (product name: Zephacus (registered trademark) tablets, production and sales company: Graxo Sumiscrain Co., Ltd.). The background of the above two groups of patients is shown in Table 1...
Embodiment 2
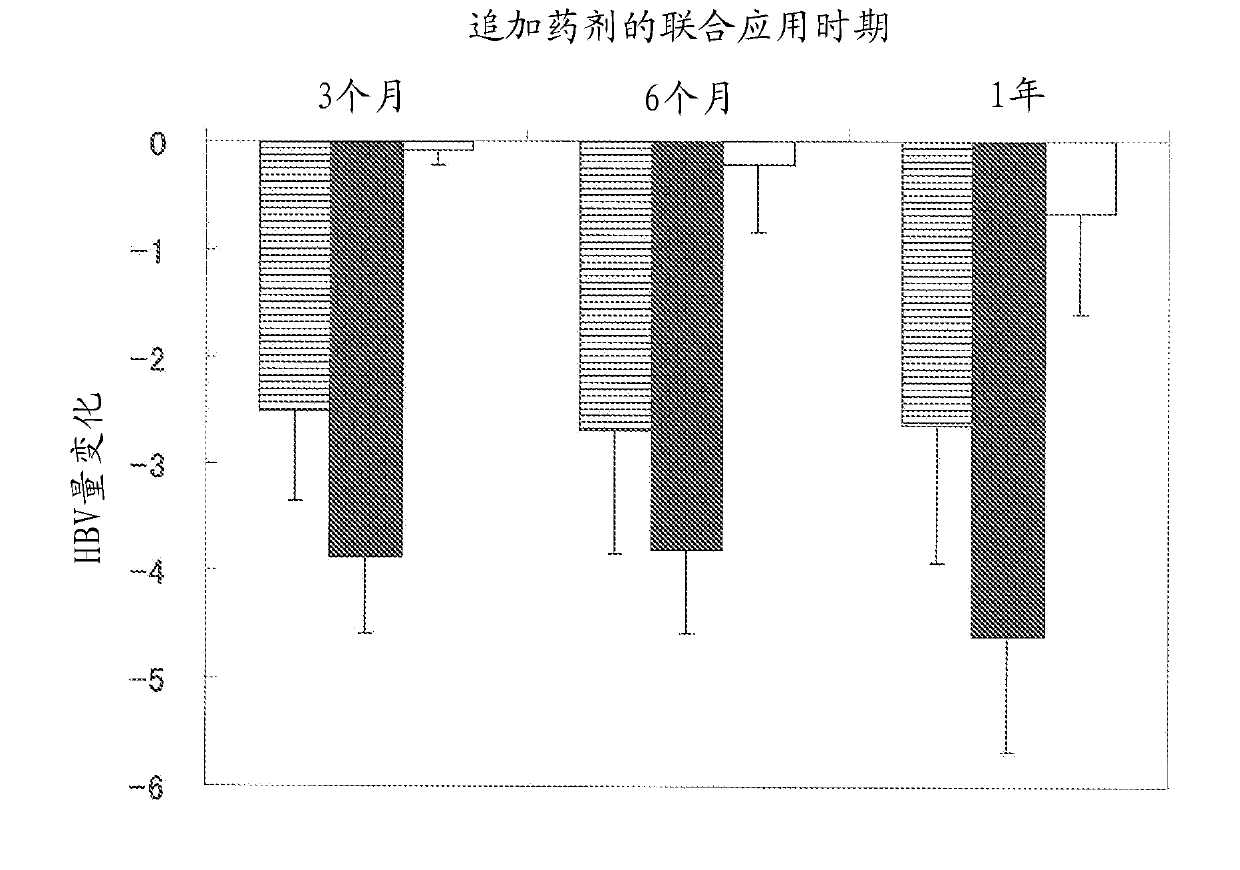
[0123] Embodiment 2: the joint application effect (2) of nucleic acid analog preparation and activity enhancer of the present invention
[0124] As rivacte granule and / or antiviral agent, administration is selected from lamivudine (Zefix (registered trademark), Graxosum Crain Co.), entecavir hydrate (Barakruud (registered trademark), Bristol Myayer Co., Ltd.) and Pivoad Nucleic acid analog preparations of Fovir (ヘプセラ (registered trademark), ヘプサスミスクライン Co., Ltd.) were used for 3 to 12 months, and the amount of hepatitis B virus in serum was measured by nucleic acid amplification detection in 20 patients with hepatitis B cirrhosis.
[0125] In addition, the above-mentioned 20 patients were patients with liver cirrhosis, and no liver cancer occurred.
[0126] Nucleic acid amplification detection is carried out by either PCR method or TMA method according to the virus amount of the patient (measurement range: 2.6-7.6 log copies / mL (PCR method), 3.7-8.7 LGE / mL (TMA method) ). LGE...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com