Non-noble metal-catalyzed polymer fibrous membrane hydroborate fuel cell
A polymer fiber and borohydride technology, applied in fuel cells, fuel cell parts, battery pack parts, etc., can solve the problems of proton exchange membrane liquid fuel leakage, high battery cost, and high cost of DBFC , to achieve the effect of suitable ion pass rate, low price and small internal resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The electrode preparation process is as follows:
[0021] Anode preparation: anode catalysts include CoO, Co(OH) 2 , hydrogen storage alloys, Au and Au alloys, Pt and Pt alloys, one or more of them, with a load of 0.1-200mg cm -2 . The anode catalyst and binder are made into a paste, evenly coated on foamed nickel or carbon paper or carbon cloth, dried in a vacuum and pressed to form a hydrogen electrode, and activated by immersing in a mixed fuel solution before testing.
[0022] There are two options for preparing the cathode:
[0023] 1) Option 1:
[0024] The cathode is composed of a waterproof and breathable layer, a catalyst layer, and a current-collecting layer. The preparation process is as follows:
[0025] The carbon cloth or carbon paper used as the current-collecting layer is treated with 5-60% PTFE hydrophobic treatment, dried, and kept in a muffle furnace at 340°C for 30 minutes to obtain the required waterproof and breathable layer. The cathode cata...
Embodiment 1
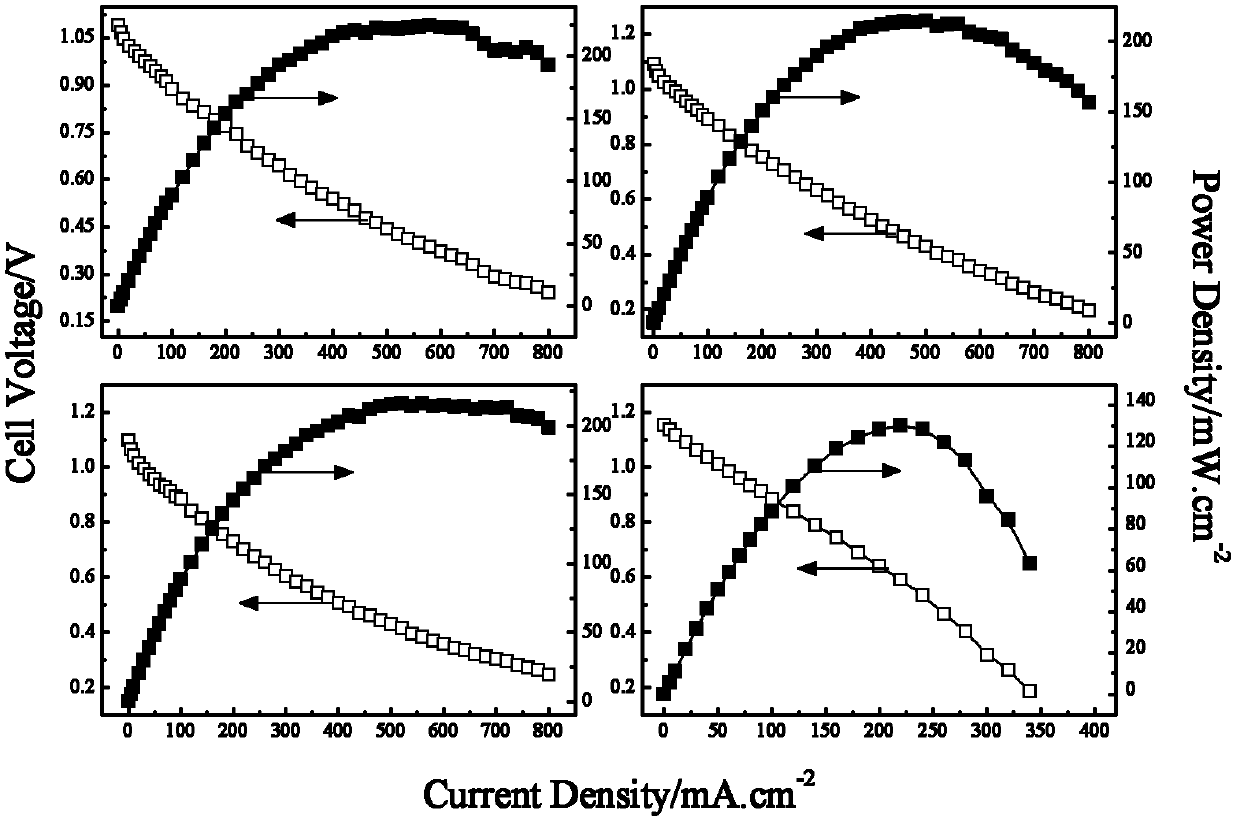
[0030] Reference attached figure 2 Shown, respectively (a) La 2 o 3 , (b) CeO 2 , (c)MnO 2, (d) FePc as the cathode catalyst, CoO as the anode catalyst, the discharge curve of the polymer fiber membrane borohydride fuel cell.
[0031] Mix 70mg of anode catalyst CoO and 10mg of binder 30% PTFE, add an appropriate amount of absolute ethanol, adjust it into a paste, and evenly coat it on the nickel foam. After vacuum drying at 80°C, press it into an electrode of 0.6mm, that is The anode was obtained and activated in the mixed fuel solution for 1 h before testing.
[0032] 30% of the cathode catalyst (respectively La 2 o 3 , CeO 2 , MnO 2 , one of FePc), 45% carbon nanotubes, 25% polytetrafluoroethylene and mix evenly, add an appropriate amount of absolute ethanol to disperse, stir to make it into a ball, and coat the ball on the nickel foam Serve and dry. The nickel foam coated with the catalyst and the waterproof and breathable membrane are rolled on a roller machine ...
Embodiment 2
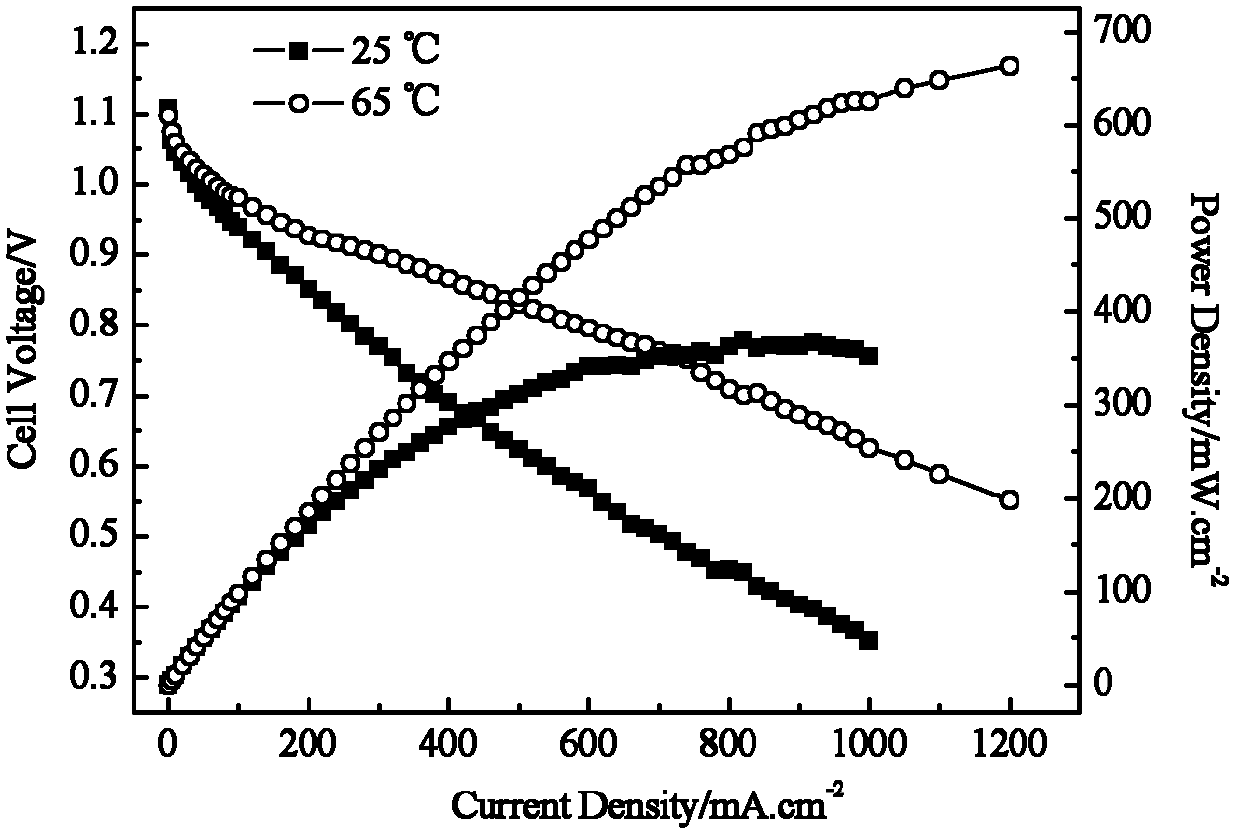
[0035] Reference attached image 3 shown in LaNiO 3 Discharge curves of polymer fiber membrane borohydride fuel cells as cathode catalyst and CoO as anode catalyst.
[0036] The cathode and anode preparation process is as described in Example 1.
[0037] Fuel is 0.8M KBH 4 +6M KOH mixed solution. The cathode oxidant is pure oxygen. Oxygen flow rate is 20ml min -1 . Operating temperature: 25, 60°C. Its maximum output power can reach 350mW cm -2 、663mW cm -2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com