Preparation method for amifostine
A technology of amifostine and aminopropylamino, which is applied in the field of drug preparation, can solve the problems of low yield, long reaction time, and difficult control, and achieve the effects of high yield, short reaction time, and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
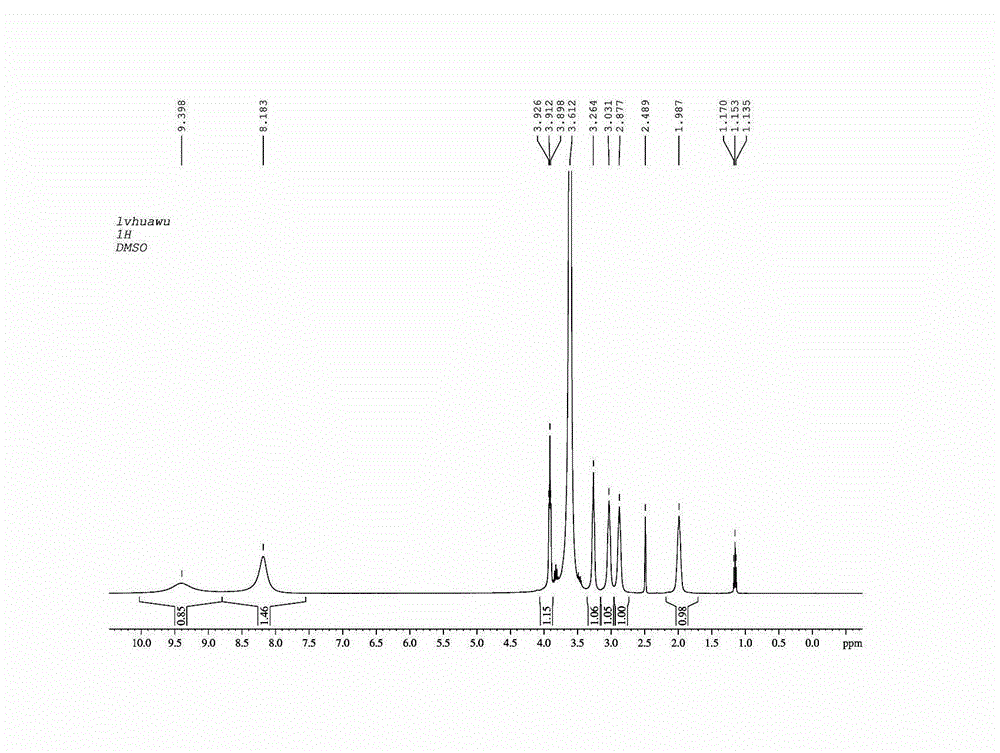
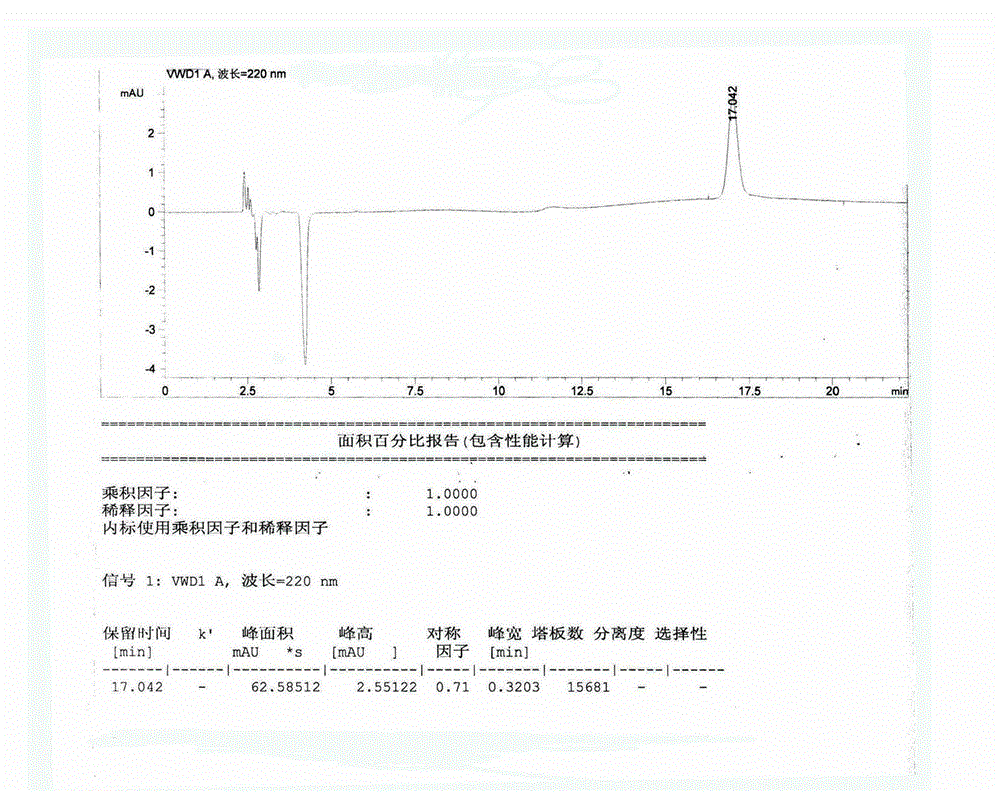
Image
Examples
Embodiment 1
[0034] The preparation method of amifostine of the present invention, the detailed steps of this preparation method are as follows:
[0035]a. Preparation of ammonium salt: First, put the basic raw material N-(2-hydroxyethyl)-1,3-propylenediamine into the reaction vessel, and add concentrated hydrochloric acid (mass percent of concentrated hydrochloric acid) dropwise under constant stirring. The concentration is 36%), the molar ratio between N-(2-hydroxyethyl)-1,3-propanediamine and concentrated hydrochloric acid is 1:2, after the concentrated hydrochloric acid is added dropwise, at 40°C Under the conditions of reaction for 60 minutes, after the reaction, the reaction solution was concentrated to dryness, and after concentration, crystallization was carried out with ethanol (the amount of ethanol added was 8 times the weight of the concentrated product), and after crystallization, it was dried (in an oven carry out drying, the drying temperature is 50°C, and the drying time is...
Embodiment 2
[0042] The preparation method of amifostine of the present invention, the detailed steps of this preparation method are as follows:
[0043] a. Preparation of ammonium salt: First, put the basic raw material N-(2-hydroxyethyl)-1,3-propylenediamine into the reaction vessel, and add concentrated hydrochloric acid (mass percent of concentrated hydrochloric acid) dropwise under constant stirring. The concentration is 38%), the molar ratio between N-(2-hydroxyethyl)-1,3-propanediamine and concentrated hydrochloric acid is 1:1.8, after the concentrated hydrochloric acid is added dropwise, at 60°C After the reaction, the reaction solution was concentrated to dryness, and then crystallized with ethanol (the amount of ethanol added was 6 times the weight of the concentrated product), and dried after crystallization (in an oven carry out drying, the drying temperature is 40°C, and the drying time is 8h), and N-(2-hydroxyethyl)-1,3-propanediamine hydrochloride is obtained after drying; ...
Embodiment 3
[0050] The preparation method of amifostine of the present invention, the detailed steps of this preparation method are as follows:
[0051] a. Preparation of ammonium salt: First, put the basic raw material N-(2-hydroxyethyl)-1,3-propylenediamine into the reaction vessel, and add concentrated hydrochloric acid (mass percent of concentrated hydrochloric acid) dropwise under constant stirring. The concentration is 36%), the molar ratio between N-(2-hydroxyethyl)-1,3-propylenediamine and concentrated hydrochloric acid is 1:2.5, after the concentrated hydrochloric acid is added dropwise, at 30°C Under the conditions of reaction for 60min, after the reaction, the reaction solution was concentrated to dryness, and after concentration, crystallization was carried out with ethanol (the amount of ethanol added was 9 times the weight of the product obtained after concentration), and after crystallization, it was dried (in an oven carry out drying, the drying temperature is 60°C, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com