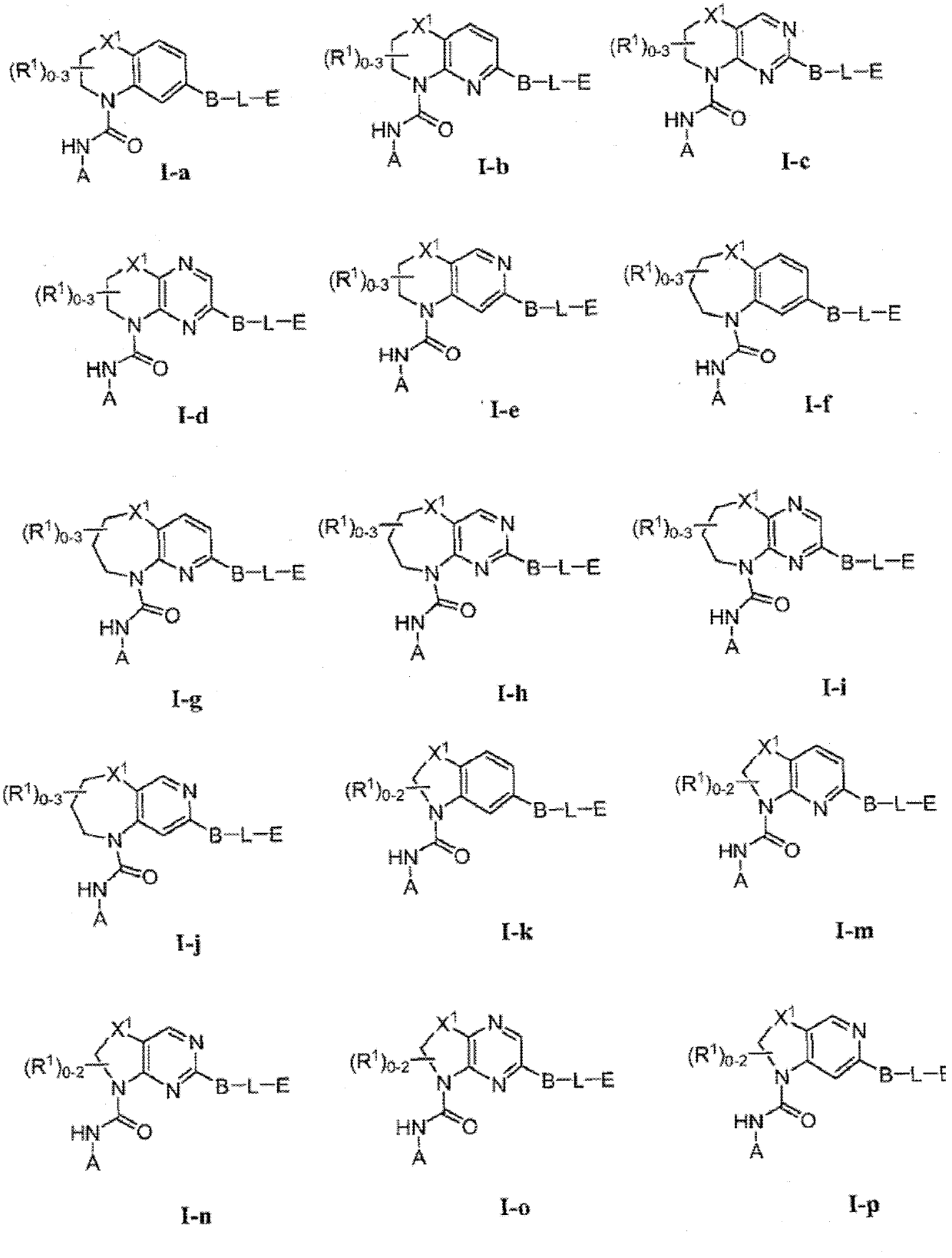
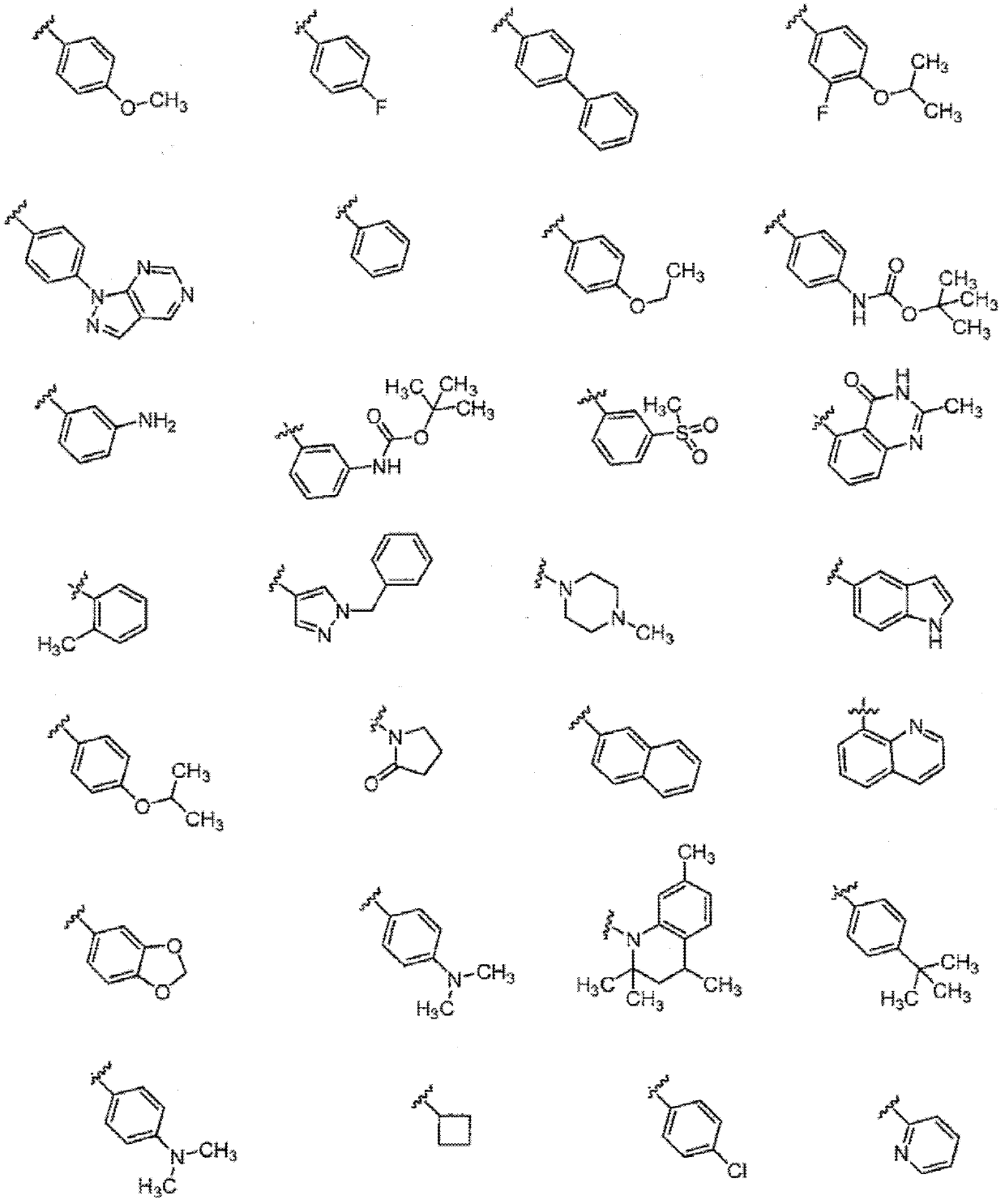
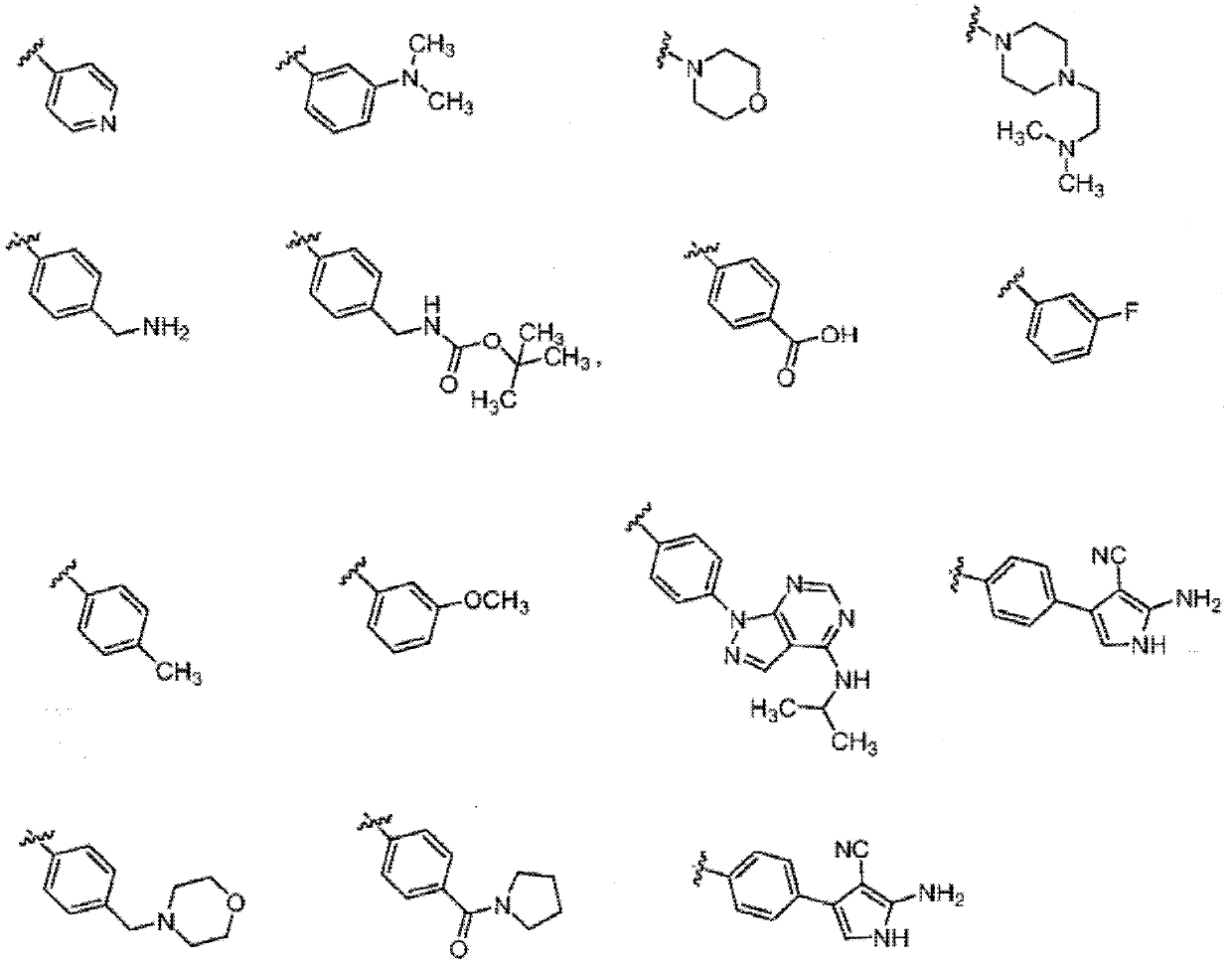
Compounds and methods of use
A kind of compound and fusion technology, applied in the direction of drug combination, organic chemistry, pharmaceutical formulation, etc., can solve problems such as weak binding and cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0236] 2-(1-(Benzo[d]thiazol-2-ylcarbamoyl)-1,2,3,4-tetrahydroquinolin-7-yl)-5-(4-methoxyphenyl) Synthesis of thiazole-4-carboxylic acid (1):
[0237]
[0238] step 1 : Preparation of 3-(4-bromophenyl)propanoic acid (1A).
[0239]
[0240] 2,2-Dimethyl-[1,3]dioxane-4,6-dione (120g, 0.84mol) and 4-bromo-benzaldehyde (153.4g, 0.84mol) in 300mL TEA and dissolved in acetic acid. The reaction mixture was refluxed overnight. At this point, the reaction mixture was diluted with water (4 L) and acidified with 6M HCl (1 L) until pH=2. The precipitated solid was filtered off, washed with dilute HCl, then dissolved in 5% NaHCO 3 dissolved in the solution. The aqueous brine was washed with ether (4 x 500 mL), filtered and acidified with dilute HCl. It was then filtered and dried in vacuo to afford 100 g (67%) of the desired product 3-(4-bromophenyl)propanoic acid (1A): 1 H NMR (DMSO-d6, 400 MHz), δ 2.5 (m, 2H), 2.8 (m, 2H), 7.2 (m, 2H), 7.5 (m, 2H), 12.2 (s, 1H).
[0241] ...
Embodiment 2
[0282] Synthesis of N-(thiazol-2-yl)-1H-imidazole-1-carboxamide (2).
[0283]
[0284] The title compound N-(thiazol-2-yl)-1H-imidazole-1-carboxamide (2) was prepared by: N-(thiazol-2-yl)-1H-imidazole-1-carboxamide (2) according to Prepared in a similar manner as described in Example 1, except that 2-aminothiazole was used in place of 2-aminobenzothiazole in step 13 of Example 1. LC / MS (APCI): m / z 195.0 (M+H).
Embodiment 3
[0286] Synthesis of N-(5-methylthiazol-2-yl)-1H-imidazole-1-carboxamide (3).
[0287]
[0288] The title compound N-(5-methylthiazol-2-yl)-1H-imidazole-1-carboxamide (3) was prepared by: N-(5-methylthiazol-2-yl)-1H-imidazole- 1-Carboxamide (3) was prepared in a similar manner as described in Example 1, except that 2-amino-5-methylthiazole was used in place of 2-aminobenzothiazole in step 13 of Example 1. LC / MS (APCI): m / z 209.2 (M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com