Compositions and methods for treatment of renin-angiotensin aldosterone system (raas)-related disorders
A compound and drug technology, used in the field of compounds for treating a nephrogenin-angiotensin-aldosterone system-related disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0226] Example 1 - Exemplary Compound Synthesis Scheme
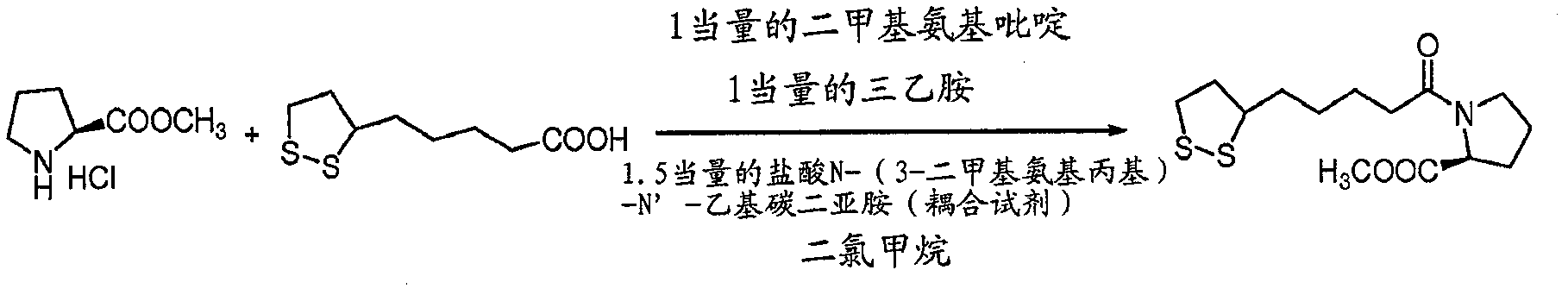
[0227] To synthesize the compounds described in the present invention, a general synthetic approach is utilized, wherein the synthetic approach includes three basic steps. In the first step, a coupling reaction is performed to link a nitrogen-containing pyrrolidine ring structure with the carboxyl group of lipoic acid to form an inert amide bond. Suitable coupling reagents that can be used in this step of the described procedure include: EDCI (N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride); DCC (dicyclohexylcarbodiimide); and CDMT (2-chloro-4,6-dimethoxy-1,3,5-triazine). Also, in this first step, the base used may include dimethylaminopyridine, triethylamine; and pyridine. Suitable chlorinated organic solvents that can be used in the first step include: methylene chloride (methylene chloride), chloroform, carbon tetrachloride, dichloroethane, and tetrachloroethane.
[0228] Briefly, said first step in...
Embodiment 2
[0231] Embodiment 2——1-(6,8-dimercaptooctanoyl) pyrrolidine-2-carboxylic acid synthesis
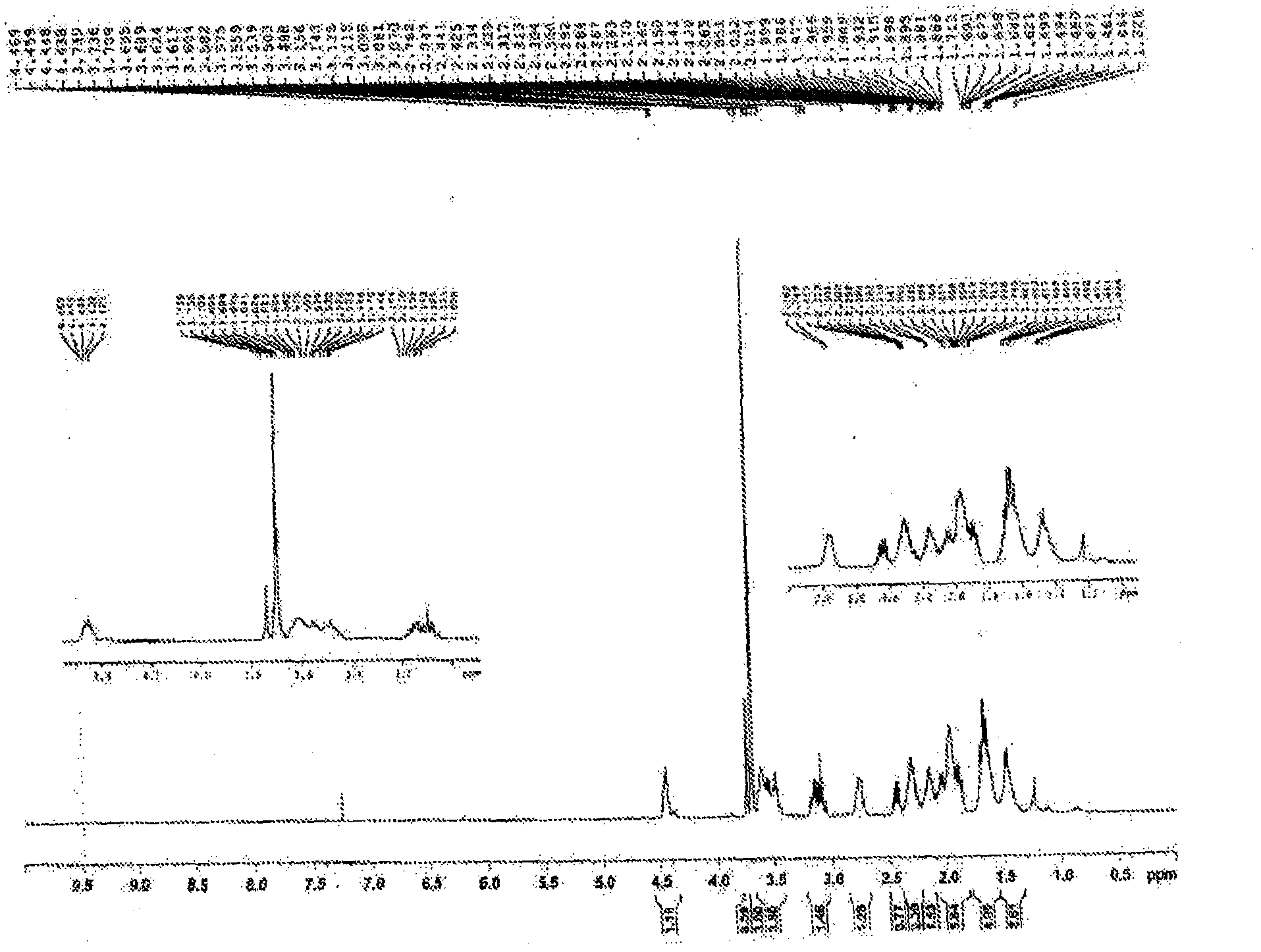
[0232] The synthesis of the 1-(6,8-dimercaptooctanoyl)pyrrolidine-2-carboxylic acid is achieved through a three-step operation in which optically active L-proline methyl ester and alpha lipoic acid as the starting point. as in the attached figure 1 L-prolyl methyl ester lipoic acid was synthesized by coupling L-prolyl methyl ester to lipoic acid using a suitable coupling reagent as shown in . Briefly, the first steps involved in the described reactions were carried out under a nitrogen atmosphere. First mix 0.206 g (1 mmol) of lipoic acid, 0.166 g (1 mmol) of L-proline methyl ester hydrochloride, and 1 equivalent of dimethylaminopyridine (DMAP) in a 100 ml round bottom flask. 0.122 g (1 mmol), and 0.101 g (0.140 ml) of triethylamine. The contents of the flask were dissolved in methylene chloride (40 mL) and stirred thoroughly at room temperature for 10 minutes. Add 0.287 g (1.5 mmo...
Embodiment 3
[0238] Embodiment 3——1-(6,8-dimercaptooctanoyl) piperidine-2-carboxylic acid synthesis
[0239] The synthesis of the described 1-(6,8-dimercaptooctanoyl)piperidine-2-carboxylic acid is realized through a three-step operation process, wherein the operation process is obtained from the The synthesis of (DL)-piperidinecarboylmethyl lipoic acid started with (DL)-piperidinecarboylmethyl ester and (DL)-α-lipoic acid. Briefly, as in the attached Figure 9 As shown in , (DL)-piperidinecarboxymethyl ester sulfide is accomplished by mixing (DL)-piperidinecarboxylate with (DL)-α-lipoic acid using a suitable coupling reagent. Caprylic acid synthesis. The first steps involved in the described reactions were carried out under nitrogen atmosphere. In a 100 ml round bottom flask, 0.206 g (1 mmol) of (DL)-α-lipoic acid, 0.166 g (1 mmol) of (DL)-methyl piperidinecarboxylate hydrochloride, dimethyl Aminopyridine 0.122 g (1 mmol), and triethylamine 0.101 g (1 mmol, 0.140 mL) were dissolved ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com