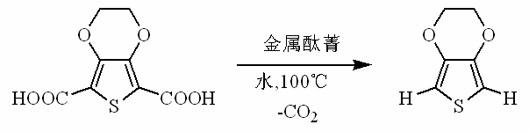
Decarboxylation method of 3,4-ethylenedioxy thiophene
A technology for decarboxylation of ethylenedioxythiophene, applied in organic chemistry, chemical recovery, etc., can solve the problems of high cost of decarboxylation, unfriendly environment, high operation requirements, etc., and achieve the effect of low cost, environmental friendliness and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
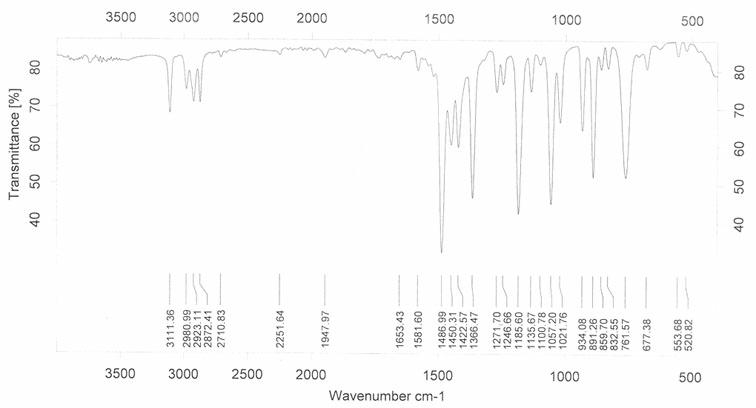
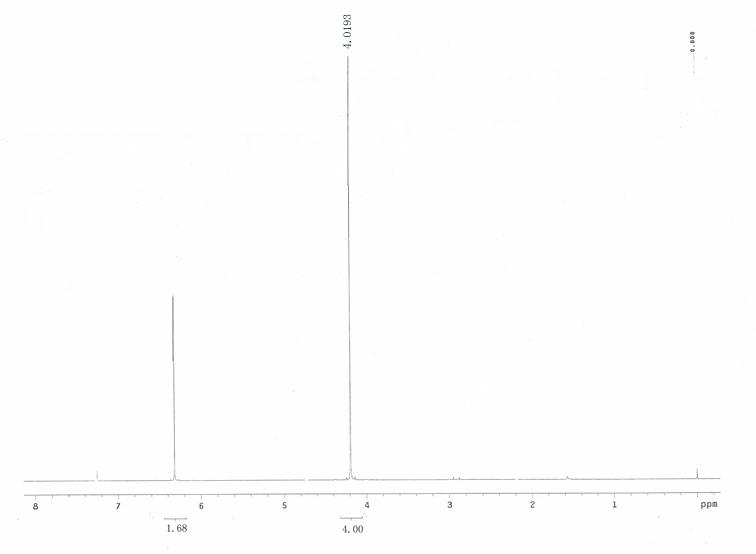
[0021] Such as figure 1 As indicated, weigh 23g 2,5-dicarboxylic acid-3,4-ethylenedioxythiophene and 0.2 g copper phthalocyanine ( figure 2 As shown, R=H, M=Cu), add it to a 250mL reaction bottle, add 100mL water, install an oil-water separator, heat to reflux, and an oily substance will separate out during the reaction. The separated oil was dried and distilled under reduced pressure to obtain 13.0 g of 3,4-ethylenedioxythiophene (92% yield). Product characterization results such as Figure 2-4 As shown, GC-MS: m / z =142, 127, 116, 95, 69, 58, 45; 1 H NMR (CDCl 3 ) δ (ppm): 6.320 (s, 2H, ArH), 4.193 (s, 4H, OCH 2 ); -1 .
Embodiment 2
[0023] Similar to Example 1, using different metal phthalocyanines as catalysts, the results are shown in the table below.
[0024]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com