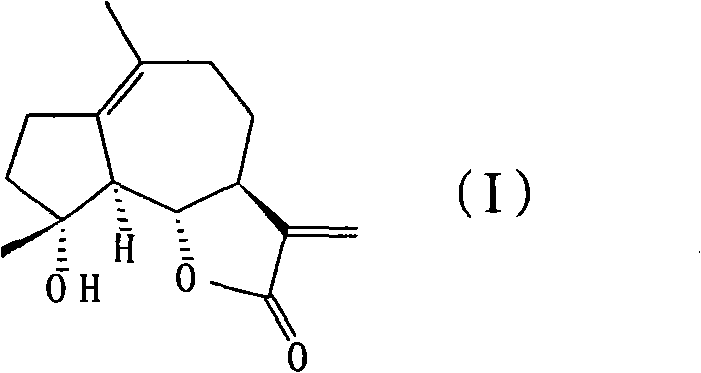
Preparation method and application of sphaelactone
A technology of lactone and parthenolide, applied in the field of medicine, can solve the problems of many side reactions, flammability of boron trifluoride, high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of laughing lactones
[0027] At room temperature, 500 mg of parthenolide and 34 mg of p-toluenesulfonic acid were dissolved in 50 mL of toluene, and the reaction was refluxed for 12 hours. The reaction solution was poured into an appropriate amount of ice water, extracted with ethyl acetate, and then the ethyl acetate layers were separated. Wash with 200 mL of 5% sodium bicarbonate and 300 mL of saturated brine, then dry with anhydrous sodium sulfate, and concentrate under reduced pressure to obtain a crude product containing laugholide, which is then subjected to silica gel column chromatography (petroleum ether:ethyl acetate=80:20 ) to obtain 300 mg of laughter, the yield is 60%.
[0028] The spectral data of the prepared laugholide are as follows:
[0029] 1 H NMR (CDCl 3 , 400MHz)δ6.14(d, J=3.2Hz, 1H), 5.45(d, J=2.8Hz, 1H), 3.76(t, J=10.4Hz, 1H), 2.61-2.68(m, 3H), 1.98-2.36 (m, 6H), 1.67-1.77 (m, 2H), 1.62 (s, 3H), 1.28 (s, 3H); 13 C NMR...
Embodiment 2
[0031] Example 2: Pharmacological effects of laughing lactones
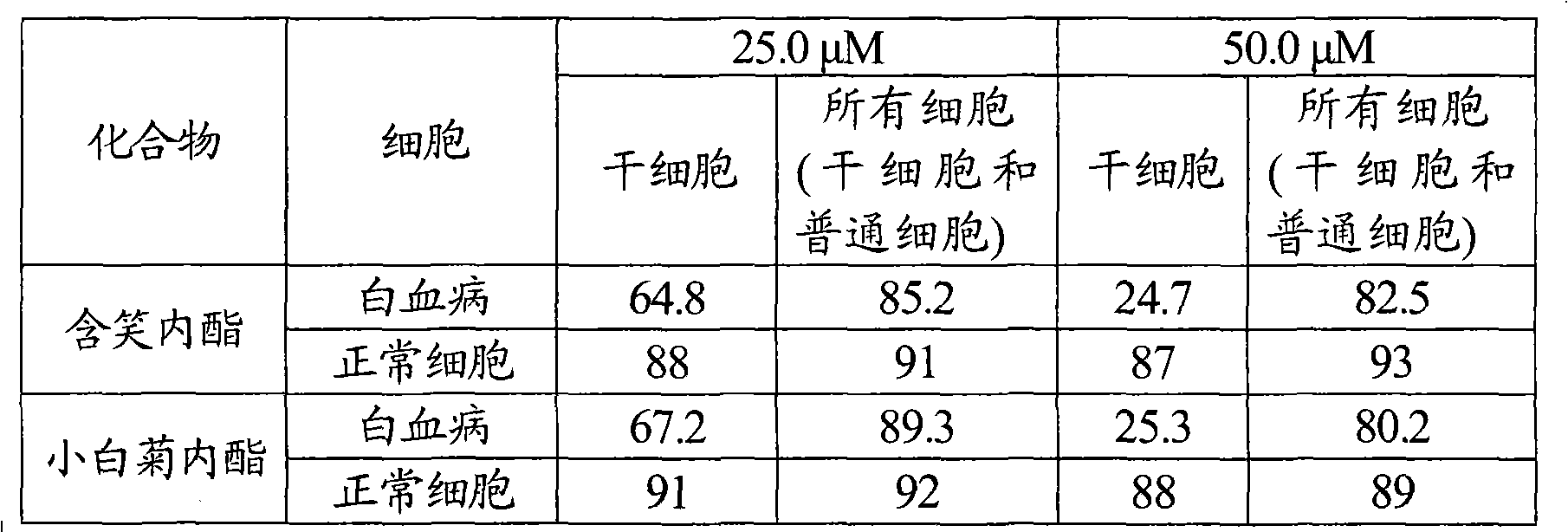
[0032] 2.1 In vitro cell test: Take the cancer cells marked by leukemia stem cells, and make the cells into 2 × 10 5 / mL cell suspension was added to the 96-well round-bottom cell culture plate, and parthenolide (positive control) and smilolide (product prepared in Example 1) were added respectively, so that the concentrations were both: 25.0 μM and 50.0 μM , 5 wells for each test concentration, set at 37°C, 5% CO 2 Incubate for 18 hours under saturated humidity conditions, and measure the absorbance (A) value at 570nm wavelength of enzyme-linked detector by MTT method. The inhibitory effect of the compounds of the present invention on leukemia cells was calculated.
[0033] Table 1 Survival rate (%) of cancer stem cells and normal cells after adding smilide
[0034]
[0035] The results of the activity test showed that the killing rate of laugholide on leukemia stem cells was comparable to that of the posi...
Embodiment 3
[0040] Example 3: Injection containing laugholide
[0041] The smilide prepared in Example 1 was dissolved with a small amount of DMSO, and water for injection was added conventionally, finely filtered, potted and sterilized to prepare an injection solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com