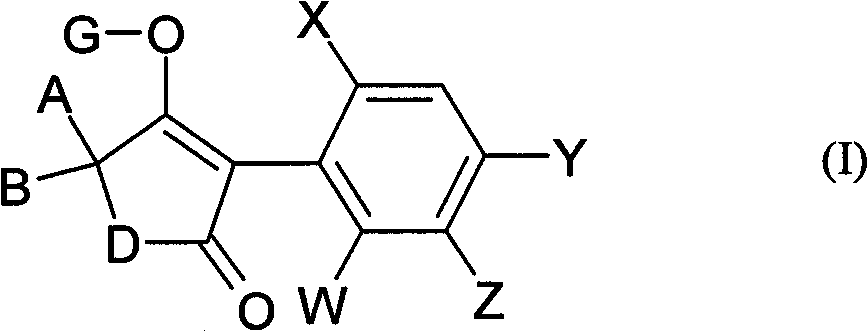
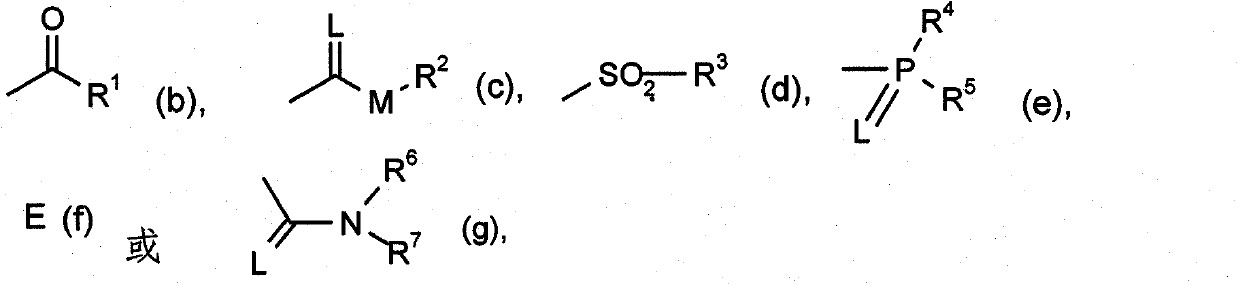
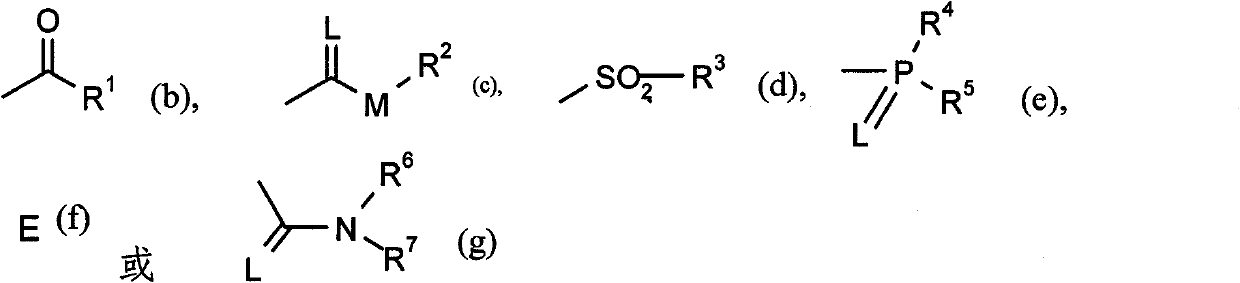
Active substance combinations having insecticidal and acaricidal properties
A technology of compounds and complexes, applied in acaricides, organic chemistry, biocides, etc., can solve problems such as poor effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0200] Green peach aphid (Myzus persicae) test
[0201] Solvent: acetone of 78 parts by weight
[0202] 1.5 parts by weight of dimethylformamide
[0203] Emulsifier: 0.5 parts by weight of alkyl aryl polyglycol ether
[0204] To prepare a suitable active compound preparation, 1 part by weight of active compound is mixed with the stated amounts of solvent and emulsifier and diluted to the desired concentration with water containing emulsifier.
[0205] Cabbage leaves (Brassica oleracea) which are heavily infested by the peach aphid (Myzus persicae) are treated by spraying with an active compound preparation of the desired concentration.
[0206] After the expected time, the killing effect in % is determined. 100% means that all green peach aphids have been killed; 0% means that none of the green peach aphids have been killed. The data were entered into the Colby formula to determine the kill rate.
[0207] In this test, for example, the following active compound complexes ...
Embodiment B
[0213] Mustard leaf beetle (Phaedon cochleariae) larval test
[0214] Solvent: acetone of 78 parts by weight
[0215] 1.5 parts by weight of dimethylformamide
[0216] Emulsifier: 0.5 parts by weight of alkyl aryl polyglycol ether
[0217] To prepare a suitable active compound preparation, 1 part by weight of active compound is mixed with the stated amounts of solvent and emulsifier and diluted to the desired concentration with water containing emulsifier. Cabbage leaves (Brassica oleracea) which are still moist and are colonized with larvae of the mustard leaf beetle (Phaedon cochleariae) are treated by spraying with the preparation of active compound of the desired concentration.
[0218] After the expected time, the killing effect in % is determined. 100% means that all beetle larvae have been killed; 0% means that none of the beetle larvae have been killed. The data were entered into the Colby formula to determine the kill rate.
[0219] In this test, the following ac...
Embodiment C
[0225] Fall armyworm (Spodoptera frugiperda) larva test
[0226] Solvent: acetone of 78 parts by weight
[0227] 1.5 parts by weight of dimethylformamide
[0228] Emulsifier: 0.5 parts by weight of alkyl aryl polyglycol ether
[0229] To prepare a suitable active compound preparation, 1 part by weight of active compound is mixed with the stated amounts of solvent and emulsifier and diluted to the desired concentration with water containing emulsifier.
[0230] Cabbage leaf leaves (Brassica oleracea), which are still moist and are colonized with larvae of the fall armyworm (Spodoptera frugiperda), are treated by spraying with the preparation of active compound of the desired concentration.
[0231] After the expected time, the killing effect in % is determined. 100% means that all caterpillars have been killed; 0% means that none of the caterpillars have been killed. The data were entered into the Colby formula to determine the kill rate.
[0232] In this test, the followi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com