Application of 2, 2, 3, 3- tetramethyl cyclopropyl carbonyl thiourea in the process for preparing antitumor medicine
A technology of tetramethylcyclopropionyl thiourea and antitumor drug, which is applied in the application field of 2,2,3,3-tetramethylcyclopropionylthiourea in the preparation of antitumor drugs, and can solve the antitumor effect No reports yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
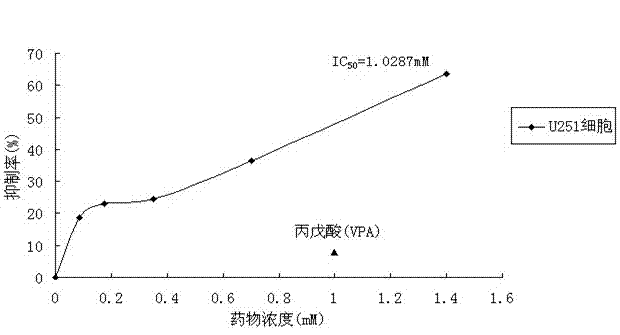
[0024] Example 1. The effect of 2,2,3,3-tetramethylcyclopropionylthiourea on the growth and proliferation of U251 cells
[0025] 1. Experimental materials and instruments
[0026] Cell line and drug: The human astroglioma cell line U251 was derived from the Sun Yat-sen University Affiliated Tumor Hospital, and 2,2,3,3-tetramethylcyclopropionylthiourea was combined by this experimental subject.
[0027] Main reagents: RPMI 1640 medium, premium fetal bovine / calf serum (FBS / CS), trypsin, dimethyl sulfoxide (DMSO), valproic acid (VPA), tetrazothiazole (MTT) powder.
[0028] Main working solution: Cell dry powder RPMI 1640 medium, double antibody storage solution (100×) (weigh 0.625g penicillin sodium, 1.000g streptomycin, add about 80ml PBS, stir well to dissolve, and dilute to 100ml. 0.22μm filter membrane Sterilize by filtration and dispense into multiple 1.5ml Ep tubes, store at -20℃, and dilute 100 times when used), phosphate buffered saline (PBS), cell digestion solution (0.25%tryp...
Embodiment 2
[0034] Example 2.2 The effect of 2,2,3,3-tetramethylcyclopropionylthiourea on the mRNA expression of U251 cell cycle factors p21WAF1 / CIP1, CyclinD1
[0035] 1. Experimental materials and instruments
[0036] Cell line and drug: The human astroglioma cell line U251 was derived from the Sun Yat-sen University Affiliated Tumor Hospital, and 2,2,3,3-tetramethylcyclopropionylthiourea was combined by this experimental subject.
[0037] Main reagents: RPMI 1640 medium, premium fetal bovine serum, PAA (stored at -20°C), trypsin, dimethyl sulfoxide (DMSO), valproic acid (VPA), TRNzol-A + Extraction reagents, Quant one-step RT-PCR kit, DEPC, EB, TBE, 6× loading buffer.
[0038] Main working solution: Cell dry powder RPMI 1640 medium, double antibody storage solution (100×) (weigh 0.625g penicillin sodium, 1.000g streptomycin, add about 80ml PBS, stir well to dissolve, and dilute to 100ml. 0.22μm filter membrane Sterilize by filtration and dispense into multiple 1.5ml Ep tubes, store at -20°C...
Embodiment 3
[0051] Example 3 Effect of 2,2,3,3-tetramethylcyclopropionylthiourea on the protein expression of HDAC3 / HDAC4 and cyclic factors p21 and CyclinD1 in U251 cells
[0052] 1. Experimental materials and instruments
[0053] Cell line and drug: The human astroglioma cell line U251 was derived from the Sun Yat-sen University Affiliated Tumor Hospital, and 2,2,3,3-tetramethylcyclopropionylthiourea was combined by this experimental subject.
[0054] Main reagents: RPMI 1640 medium, premium fetal bovine serum, PAA (stored at -20°C), trypsin, dimethyl sulfoxide (DMSO), valproic acid (VPA), nuclear protein extraction kit, p21waf1 / cip1 Antibody (I antibody), HDAC3 antibody (I antibody), HDAC4 antibody (I antibody), β-actin reference antibody (I antibody), HRP-labeled goat anti-mouse antibody (Goat anti-mouse antibody, II antibody), Protein weight Marker.
[0055] Main working solution: dry cell powder RPMI 1640 medium, double antibody storage solution (100×) (weigh 0.625g penicillin sodium, 1.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com