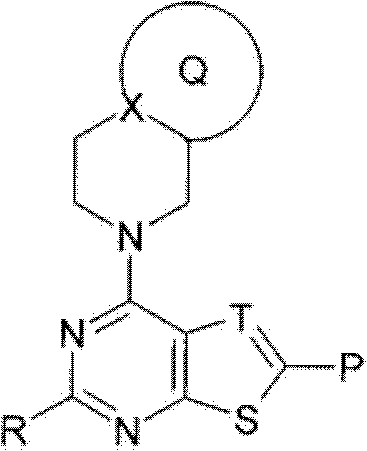
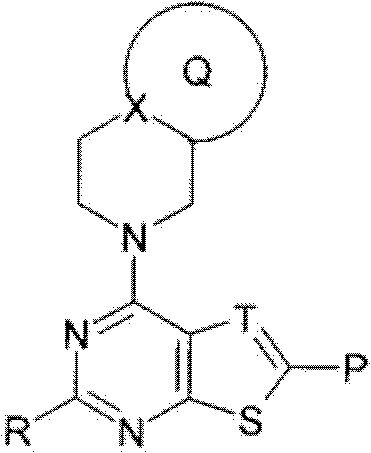
Fused heterocyclic compound
A compound and heterocyclic technology, applied in the field of platelet aggregation inhibitors, pharmaceutical compositions for inhibiting platelet aggregation, heterocyclic compounds or their pharmaceutically acceptable salts, can solve problems such as application limitations and low efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1-1-1
[0739] 2,4-Dichloro-6-propyl-thieno[2,3-d]pyrimidine
[0740]
[0741] The synthesis was performed according to known methods (WO 2006 / 079916).
preparation example 1-1-2
[0743] 3-Trifluoromethyl-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride
[0744]
[0745] Synthesis was performed according to a known method (Organic Letters 2005, 7(6), 1039-1042).
preparation example 1-1-3
[0747] 7-(2-Chloro-6-propyl-thieno[2,3-d]pyrimidin-4-yl)-3-trifluoromethyl-5,6,7,8-tetrahydro-[1,2 ,4]triazolo[4,3-a]pyrazine
[0748]
[0749] The compound (225 mg, 0.91 mmol) obtained in Preparation Example 1-1-1 and the compound (250 mg, 1.09 mmol) obtained in Preparation Example 1-1-2 were diluted in N,N-dimethylformamide (5 mL). mmol), and diisopropylethylamine (353 mg, 2.73 mmol) was added thereto, and the mixture was stirred for 16 hours. The reaction mixture was distilled under reduced pressure, diluted with dichloromethane, and washed with water. The organic layer was dried over anhydrous magnesium sulfate, distilled under reduced pressure, then solidified, and washed with diethyl ether to obtain the title compound (289 mg, 79%).
[0750] 1 H NMR (400MHz, CDCl 3 );
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com