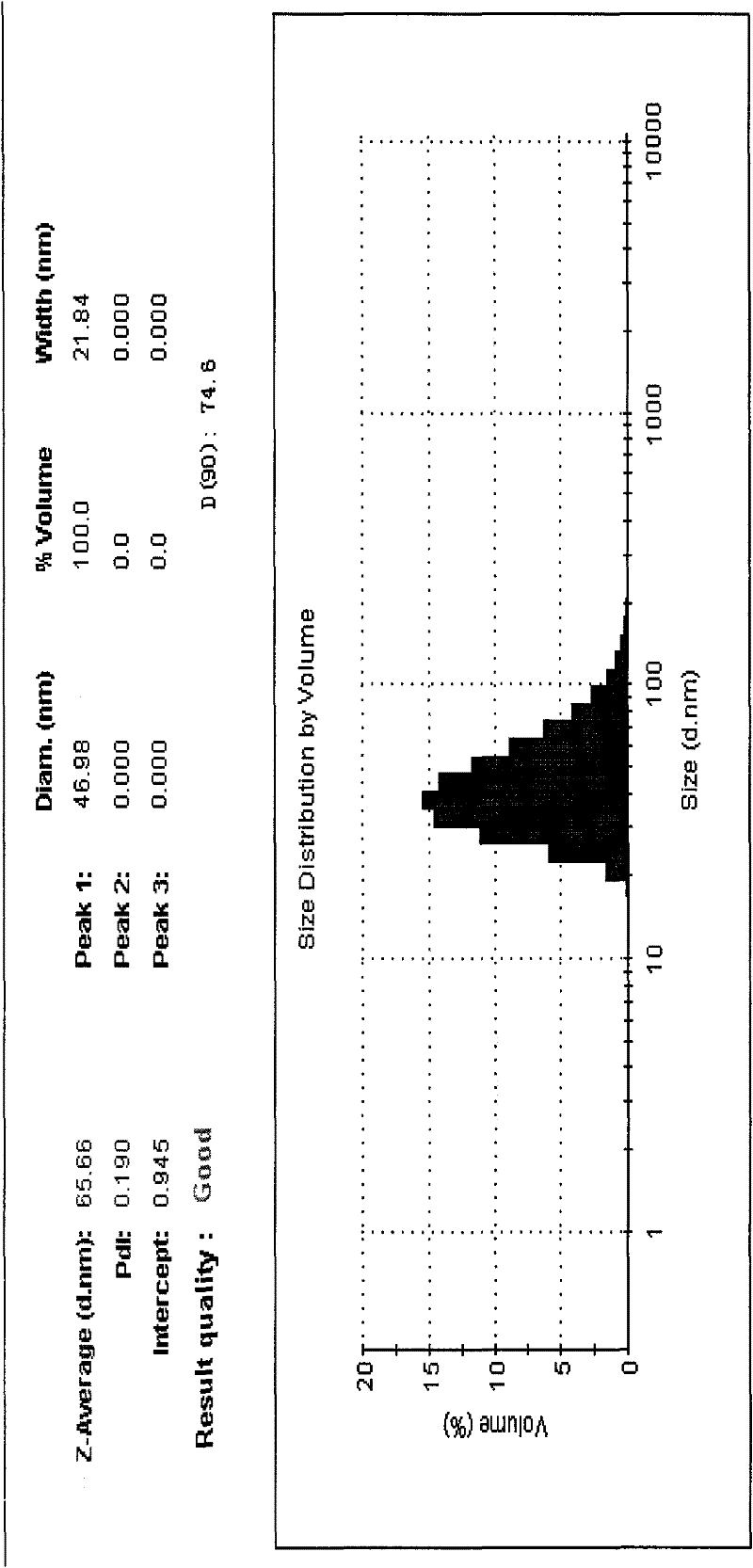
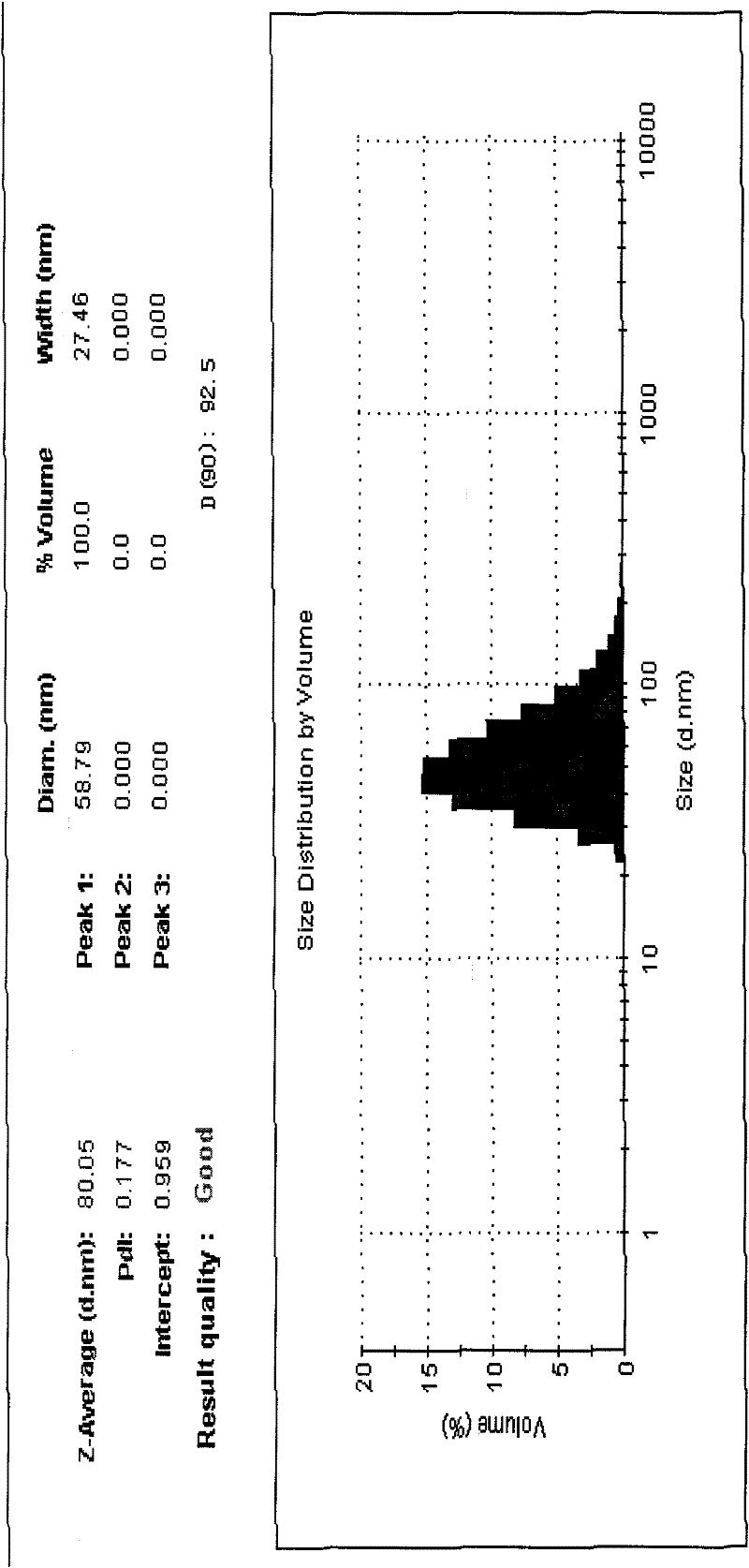
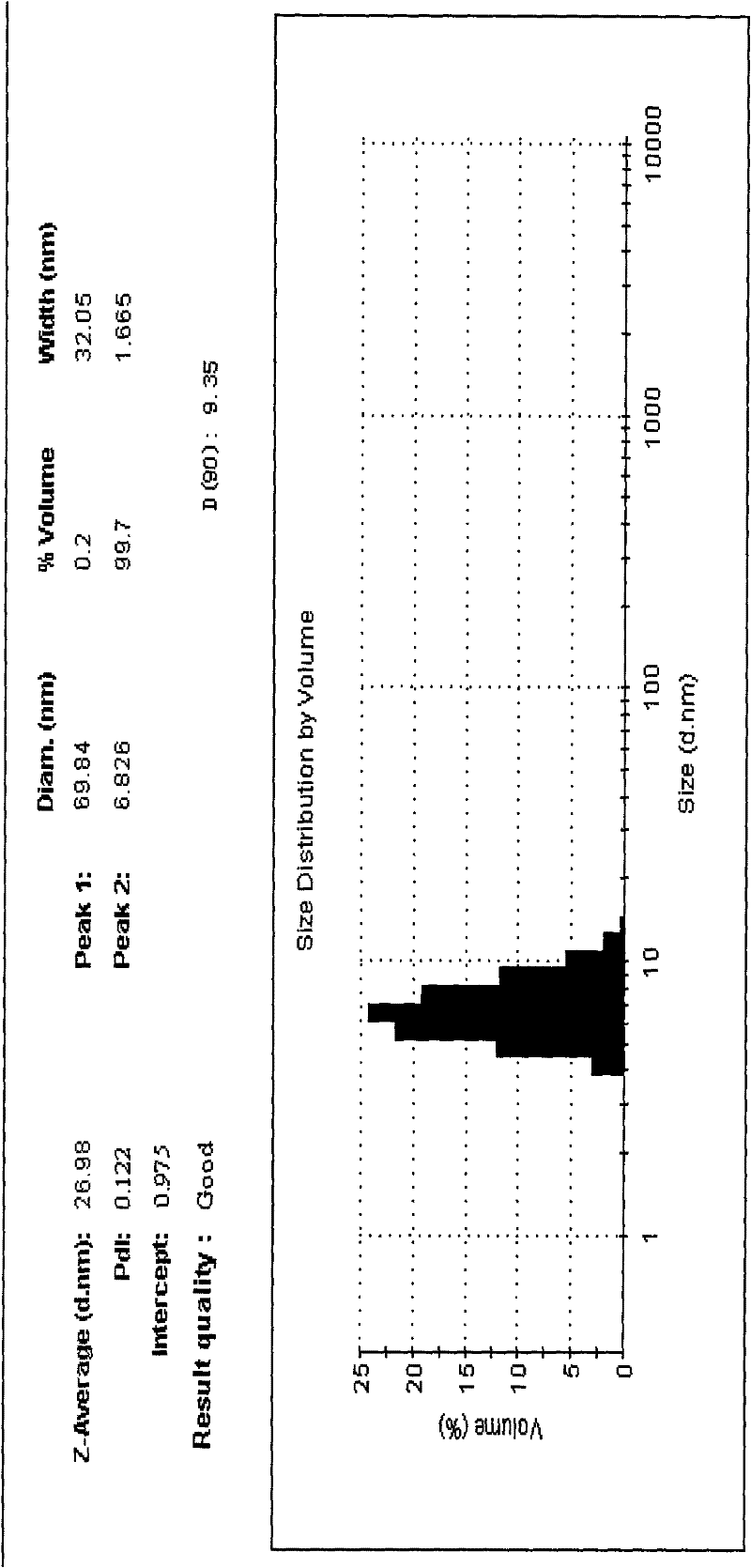
Alprostadil lipid nanosphere injection and preparation method thereof
A technology of dil lipid and alprostadil, which is used in blood diseases, pharmaceutical formulations, emulsion delivery, etc., can solve the problems of increasing blood circulation time, affecting clinical application of products, shortening pharmacological action time, etc., and reducing blood clearance. , reduce pulmonary circulation inactivation and blood clearance, reduce the effect of pulmonary circulation inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The alprostadil lipid nanosphere injection of the present invention is prepared from the following raw materials by weight: 0.03 g of alprostadil, 90 g of medium chain oil, 40 g of soybean lecithin, polyethylene glycol dodecahydroxy hard It is prepared from 50g of fatty acid ester, 20g of glycerin and 800g of water.
[0035] In a sterile workshop or a class 100 purification workshop, weigh 90 g of medium chain oil for injection, add 40 g of sterile soybean lecithin and 50 g of sterile polyethylene glycol dodecyl stearate, and heat it to 60 g -70°C, mix well, then add 30 mg of the main drug alprostadil to it to dissolve it; add a mixture of 800g water for injection at 60-70°C and 20g injection-grade glycerin, keep the temperature at 60-70°C, shear and stir to form The colostrum is then put into a high pressure homogenizer, circulated 3-4 times under the pressure of 20-100MPa, cooled to room temperature, filtered with a 0.45μm filter, and then filtered with a 0.22μm filte...
Embodiment 2
[0037] The alprostadil lipid nanosphere injection of the present invention is prepared from the following raw materials in parts by weight: 0.002 g of alprostadil, 40 g of medium chain oil, 26 g of egg yolk lecithin, polyethylene glycol dodecanol Fatty acid ester 25g, glycerin 22g, water 890g.
[0038] In a sterile workshop or a class 100 purification workshop, weigh 40 g of medium-chain oil for injection, add 26 g of sterile egg yolk lecithin and 25 g of sterile polyethylene glycol dodecyl stearate, and heat it to 60 -70°C, mix well, then add 2 mg of the main drug alprostadil to it to dissolve it, keep at 60-70°C; add a mixture of 890g water for injection and 22g injection-grade glycerol at 60-70°C, keep the temperature at 60-70°C ℃, shear and stir to form colostrum, then put it into a high-pressure homogenizer, circulate 8 times under the pressure of 20-100MPa, cool to room temperature, filter with a 0.45μm filter, and then filter with a 0.22μm filter, Prepared as an emulsi...
Embodiment 3
[0040] The alprostadil lipid nanosphere injection of the present invention is prepared from the following raw materials in proportion by weight: 0.06 g of alprostadil, 42 g of medium chain oil, 28 g of soybean oil, 30 g of soybean lecithin, and 30 g of polyethylene glycol. Dodecyl hydroxystearate 40g, glycerin 22g, water 838g.
[0041] In a sterile workshop or a class 100 purification workshop, weigh 42 g of medium chain oil for injection and 28 g of soybean oil for injection according to the weight ratio, add 30 g of sterile soybean lecithin and sterile polyethylene glycol dodecyl 40g stearate, heat it to 60-70℃, mix well, add 60mg of main drug alprostadil to it, dissolve it, keep at 60-70℃; add 838g water for injection at 60-70℃ and injection grade The mixed solution of 22g glycerol was kept at 60-70°C, sheared and stirred to form colostrum, then put into a high-pressure homogenizer, circulated 10 times under the pressure of 20-100MPa, cooled to room temperature, and used a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com