Porous solid phase for binding assay, and binding assay method using the same
一种多孔性、类固醇的技术,应用在测量装置、仪器、科学仪器等方向,能够解决没有解决测量波形扰动问题、不存在解决方案等问题,达到优异再现性、防止假阴性和假阳性、防止流动行进不良的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
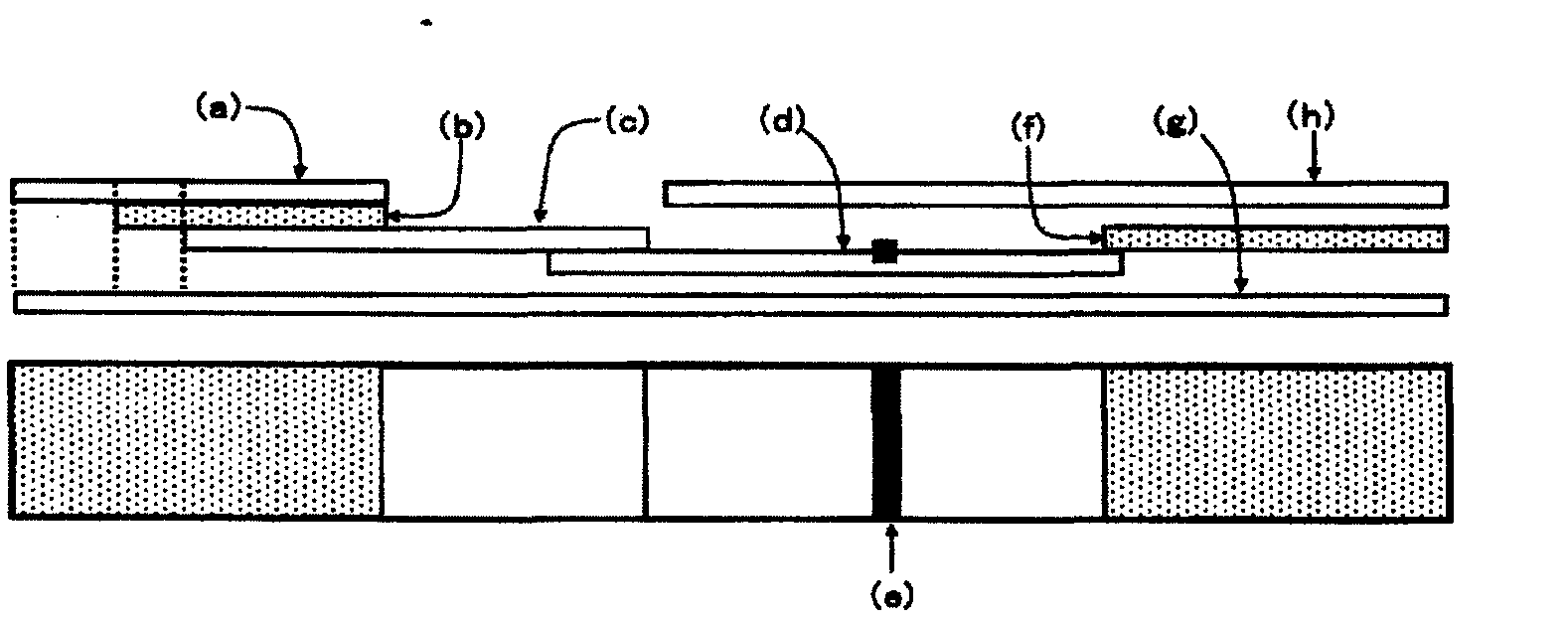
[0147] Example 1: Manufacture of an immunochromatographic device using a test strip according to the invention
[0148] (1) Preparation of the membrane of the present invention (porous solid phase for binding assay) on which anti-DD antibody is immobilized
[0149] A nitrocellulose membrane ("HF240" manufactured by Millipore) was soaked in 10 mmol / l phosphate buffer (pH 7.2) containing the surfactant of the present invention shown in Table 1 at a concentration of 0.05% (w / v), and shake at room temperature for 30 minutes. After removing excess liquid, the membrane was dried in an oven at 37°C for 1 hour. The obtained surfactant-containing membrane was then processed in the same manner as in step (3) of Comparative Example 1 to obtain the membrane of the present invention on which the anti-DD antibody was immobilized.
[0150] (2) Manufacture of the immunochromatographic device of the present invention
[0151] In the same manner as in step (5) of comparative example 1, the t...
Embodiment 2
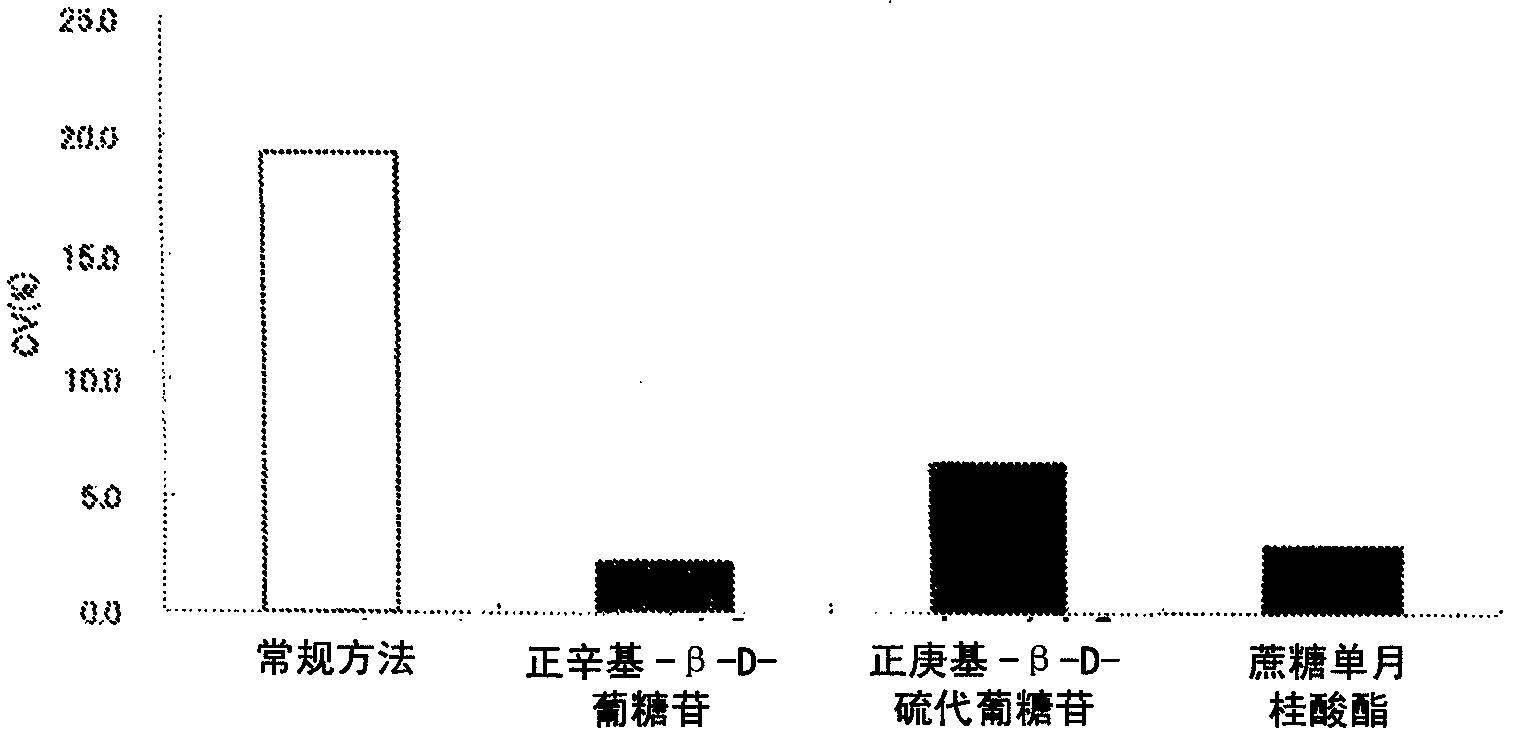
[0152] Example 2: Verification of the effect of preventing poor flow progression of test samples exhibited by the porous solid phase for binding assays according to the present invention [1]
[0153] (1) Preparation of model whole blood with high Ht value
[0154] Whole blood was centrifuged to obtain a blood cell layer. Plasma obtained from the same whole blood was added to the blood cell layer to obtain model whole blood with an Ht value of 70%.
[0155] (2) Test method
[0156] 100 μl of model whole blood was applied to the sample pad of the test strip of the device according to the invention manufactured in Example 1, and after 15 minutes, it was checked with the naked eye whether the purple-red line originating from the conjugate and indicating the edge of the advancing liquid Access to the absorbent located at one end of the test strip. If the magenta line had reached the absorber at one end of the test strip, it was judged that the porous solid phase had prevented th...
Embodiment 3
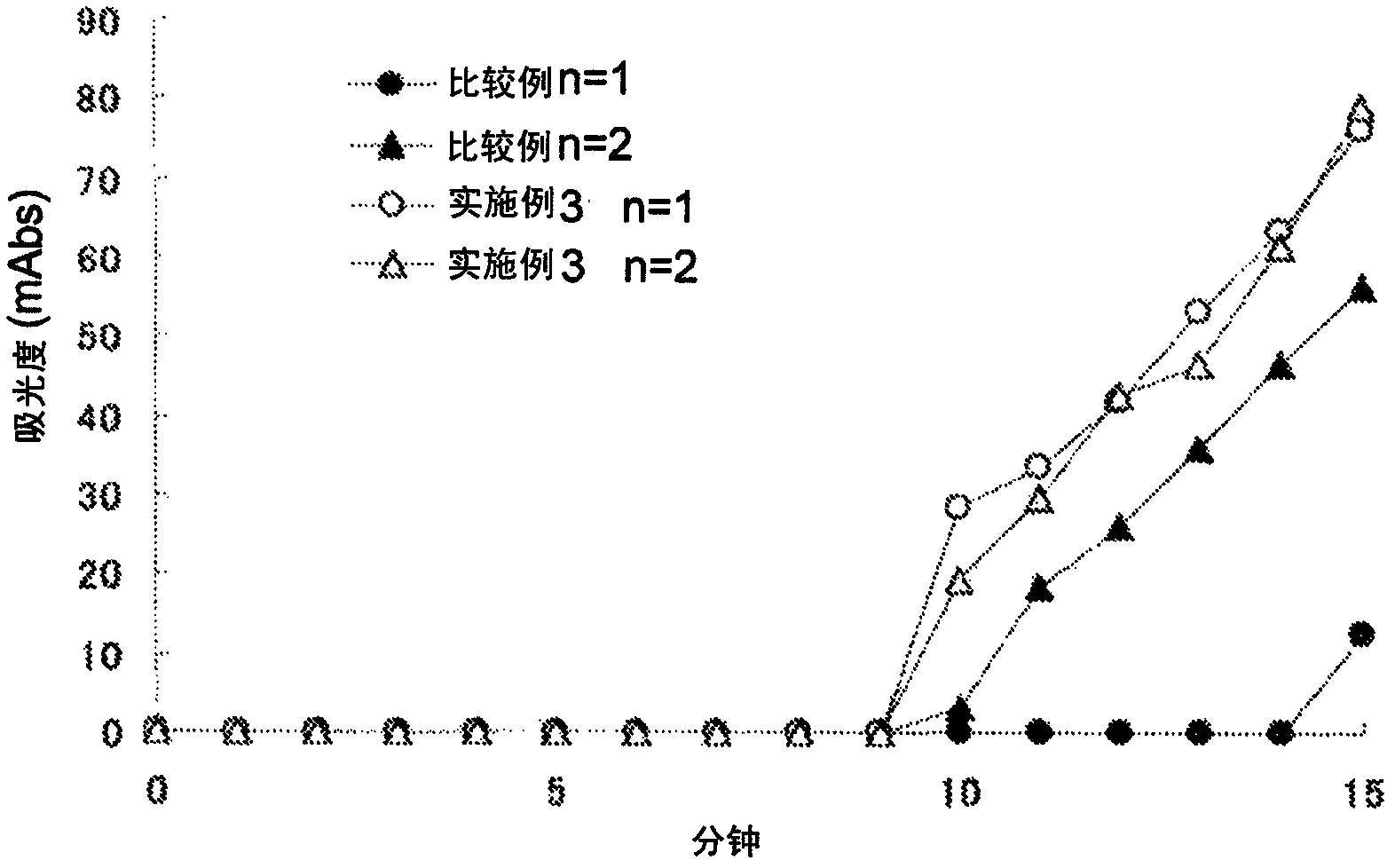
[0162] Example 3: Verification of the effect of preventing poor flow progression of test samples exhibited by the porous solid phase for binding assays according to the present invention [2]
[0163] (1) Preparation of model whole blood with high Ht value (DD model whole blood) containing purified D-dimer (DD)
[0164] Whole blood was centrifuged to obtain a blood cell layer. 10 mmol / l Tris-HCl buffer (pH 8.0) containing purified DD and plasma obtained from the same whole blood was added to the blood cell layer to obtain DD with a final concentration of 1.0 μg / ml after reconstitution and Ht values 70% model whole blood.
[0165] (2) Test method
[0166] 100 μl of DD model whole blood was applied to the sample pad of the immunochromatographic device comprising the porous solid phase for binding assay (containing n-octyl-β-D-glucoside) of the present invention manufactured in Example 1, and at intervals of 1 minute (from 1 minute to 15 minutes after adding the sample) by usin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com