5-thiazole amides and their biological applications
A technology of thiazolamide and compound, which is applied in the application field of preparing antitumor drugs, can solve the problems of decreased radiotherapy sensitivity and the like, and achieve the effect of strong proliferation inhibition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
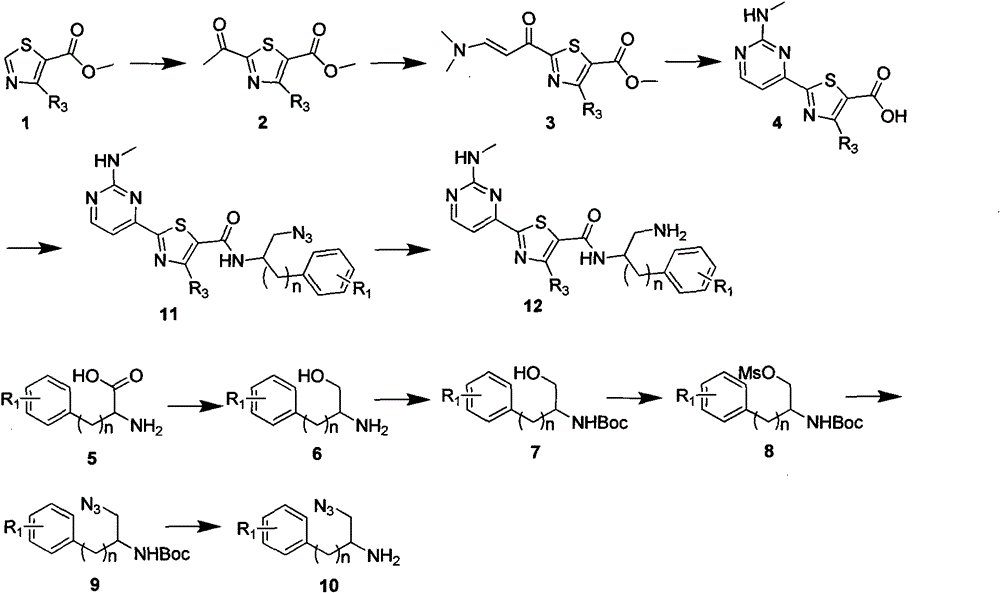
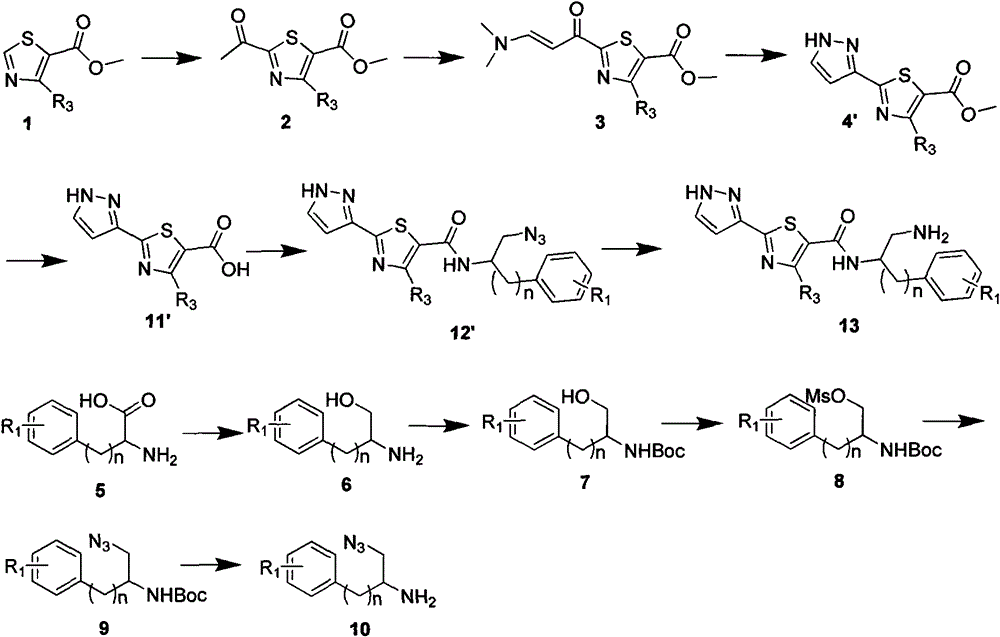
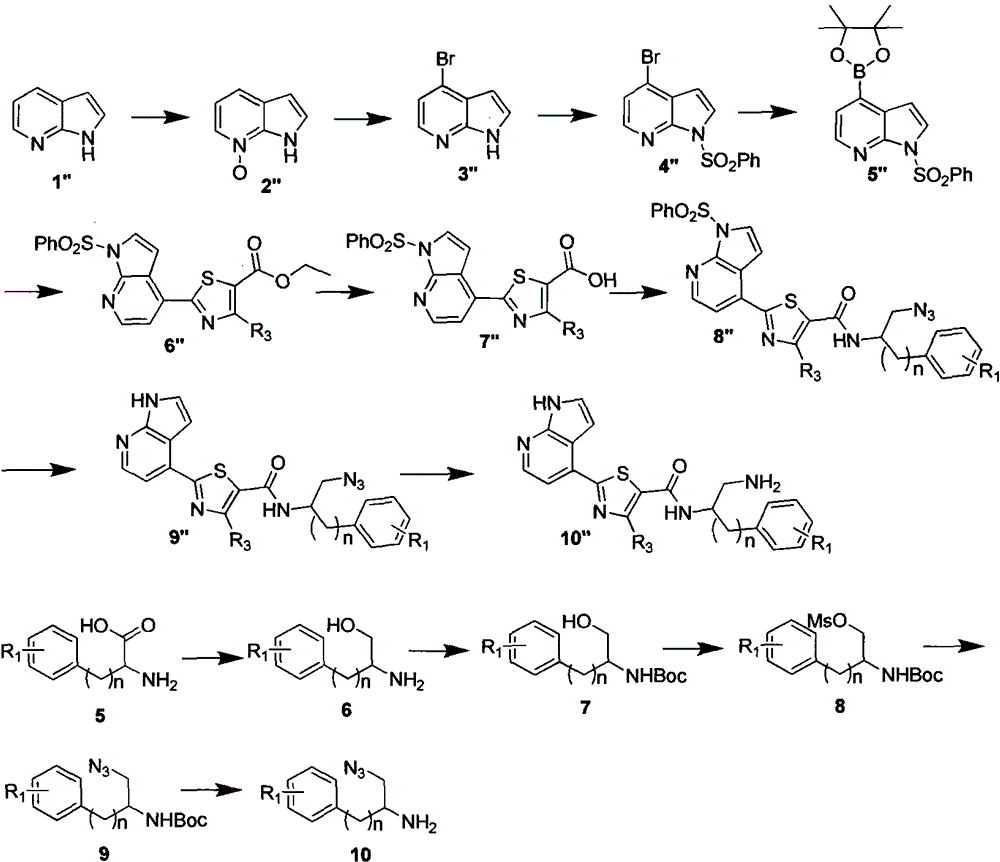
Image
Examples
example 1
[0075] (S)-N-(1-amino-3-(2,4-dichlorophenyl)propan-2-yl)-2-(2-(methylamino)pyrimidin-4-yl)thiazole-5- Carbonic acid amide
[0076] (S)-N-(1-amino-3-(2,4-dichlorophenyl)propan-2-yl)-2-(2-(methylamino)primidin-4-yl)thiazole-5-carboxamide
[0077]
[0078] 2-Acetyl-5-thiazole methyl ester (1b)
[0079] methyl-2-acetylthiazole-5-carboxylate
[0080]
[0081] Under ice bath at 0°C, 5-thiazole methyl ester 1a (2.86g, 20mmol) was dissolved in 10ml of 4M sulfuric acid solution (2.0eq), and FeSO 4 7H 2 O (33.4g, 6.0eq), 40% acetaldehyde solution (13.22g, 6.0eq), N 2 Under protection, 70% t-BuOOH (12ml, 6.0eq) was slowly added dropwise through a constant pressure funnel, and the dropwise addition was completed in 15 minutes, then transferred to room temperature for 2 hours, extracted with EA (60mlx3), saturated with FeSO 4 solution washed twice with water, saturated NaHCO 3 solution, washed once with saturated saline, anhydrous Na 2 SO 4 After drying, the solvent was evapo...
example 2
[0119] (S)-N-(1-amino-3-phenyl)propan-2-yl)-2-(2-(methylamino)pyrimidin-4-yl)thiazole-5-carbonic acid amide
[0120] (S)-N-(1-amino-3-phenylpropan-2-yl)-2-(2-(methylamino)pyrimidin-4-yl)thiazole-5-carboxamide
[0121]
[0122] MS(ESI)m / z369.1[M+H] + . 1 HNMR (400Hz, MeOH-d 4 )δ8.41(d, J=4.8Hz, 1H), 8.37(s, 1H), 7.30(t, J=4.36, 5.6Hz, 5H), 7.20-7.24(m, 1H), 4.57(m, 1H ),3.24(dd,q,J=13.08,3.44Hz,1H),3.15(dd,q,J=12.84,10.28Hz,1H),2.99(m,2H),2.96(s,3H).
[0123] (S)-N-Boc--2-Amino-3-phenyl-1-propanol (2a)
[0124] (S)-tert-butyl1-hydroxy-3-phenylpropan-2-ylcarbamate
[0125]
[0126]1M solution of Boc-Phe-OH (5.32g, 20mmol) in THF, N 2 Cool to 0°C under protection, followed by TEA (3.34ml, 1.2eq), ClCO 2 Me (1.85ml, 1.2eq), continue to stir for 25min, filter with suction, transfer the filtrate to 0°C, add 4M NaBH4 solution (7.5ml, 1.5eq) dropwise, continue to stir for 30min, transfer to room temperature, stir for 2h, 2M HCl Adjust the pH to 2, extract with EA (60mlx3...
example 3
[0129] (S)-N-(1-amino-3-(2,4-dichlorophenyl)propan-2-yl)-4-methyl-2-(2-(methylamino)pyrimidin-4-yl ) Thiazole-5-carbonic acid amide
[0130] (S)-N-(1-amino-3-(2,4-dichlorophenyl)propan-2-yl)-4-methyl-2-(2-(methylamino)pyrimidin-4-yl)thiazole-5-carboxamide
[0131]
[0132] 2-Amino-4-methyl-5-thiazole ethyl carbonate (3a)
[0133] ethyl2-amino-4-methylthiazole-5-carboxylate
[0134]
[0135] In 200ml of absolute ethanol, add ethyl 2-chloroacetoacetate (25g, 150mmol), thiourea (22.8g, 2.0eq), reflux overnight, cool to room temperature, spin off the solvent under reduced pressure, add 500ml of water, 2N NaOH The pH of the solution was adjusted to 10, and a white solid precipitated out. The stirring was continued for 10 min, filtered by suction, and dried in vacuo to obtain 3a (27.37 g, 98%). MS(ESI)m / z187.1[M+H] + . 1 HNMR (400Hz, CDCl 3 )δ5.55(br,s,2H),4.29(d,J=7.2Hz,2H),2.52(s,3H),1.34(t,J=7.2Hz,3H).
[0136] 4-Methyl-5-thiazole ethyl carbonate (3b)
[0137] ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com