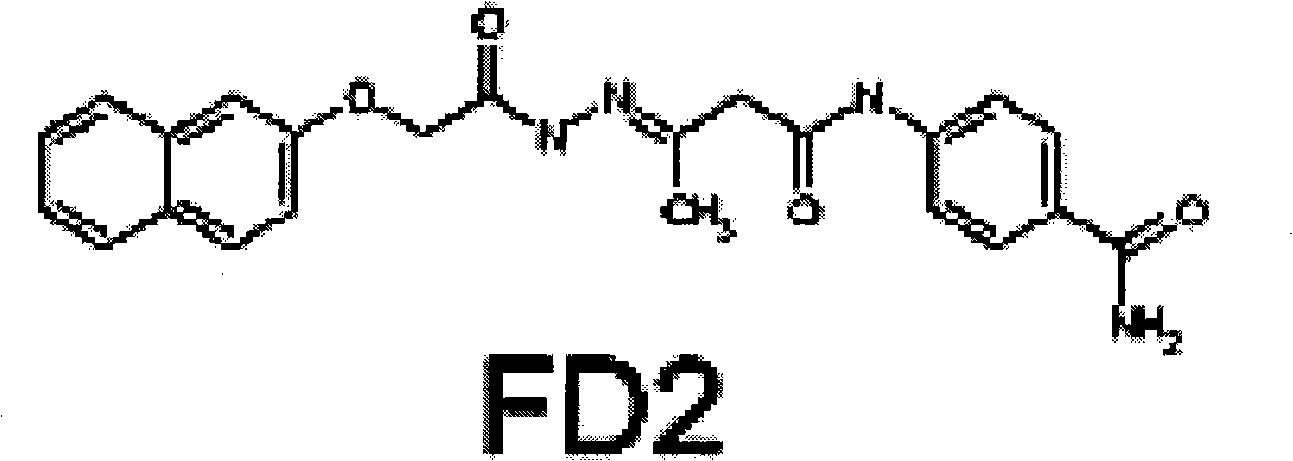
Drug application of 4-((3-(((2-naphthoxy)acetyl)hydrazo)butyryl)amine) benzamide
A technology of benzamide and acetyl, applied in the field of preparation of anti-AIDS drugs, can solve problems such as poor curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0043] Embodiment compound anti-HIV-1 virus increment test
[0044] MT-2 cells were planted in a 96-well plate, 10 per well 4 indivual. The medium is RPMI 1640 medium containing 10% fetal bovine serum. HIV-1 virus was used to infect the cells with 100 50% tissue infection equivalents, and at the same time, the compound was added with different concentration gradients and cultured overnight (the well without compound was used as the blank control). The next day, replace with fresh medium without small molecule compounds. On the fourth day, get 100uL of the culture supernatant, add 5% volume of Triton-X100 to obtain the virus lysate, and then use the ELISA method to measure the p24 protein content (p24 is the coat protein of HIV-1, which can be used as a measure of the number of virions) index of). Simply put, the double-antibody sandwich method is used to measure the amount of p24 protein produced in each well. Coat the plate with anti-HIV immunoglobulin overnight (pH 9.6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com