Process for preparation of trifluoromethanesulfonyl fluoride
A technology of trifluoromethanesulfonyl fluoride and trifluoromethanesulfonyl chloride is applied in the field of manufacture of trifluoromethanesulfonyl fluoride and can solve problems such as cost increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
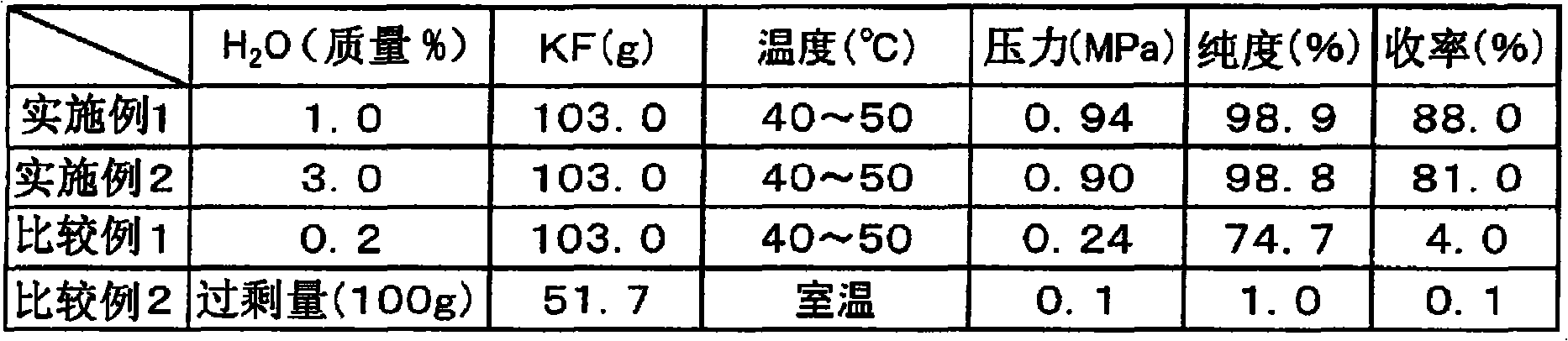
[0049] Feed 103 g (1.78 mol) of 1% aqueous potassium fluoride into a 500 mL pressure-resistant reaction vessel, cool to 5° C. with ice water, and degas under vacuum. Thereto was added trifluoromethanesulfonyl chloride (100 g (0.595 mol)). Close it and slowly raise the temperature, and stir for 4 hours at the inner temperature of the reactor at 40-50°C. At this time, the reactor pressure was 0.94 MPa. The gas in the reactor was circulated in a condenser cooled to -40°C to -20°C, and collected with a trap cooled by liquid nitrogen. 80.3 g of the captured product was obtained, and the generation of trifluoromethanesulfonyl fluoride was confirmed by gas chromatography (GC) analysis, and it was obtained with a purity of 98.9% and a yield of 88% (conversion rate 99%, selectivity 89%). (In addition, in Example 1, "1% aqueous potassium fluoride" means containing 1 mass % of water with respect to 100 mass % of potassium fluoride. Hereafter, it is the same in this specification.)
Embodiment 2
[0051] Feed 103 g (1.78 mol) of 3% aqueous potassium fluoride into a 500 mL pressure-resistant reaction vessel, cool to 5° C. with ice water, and degas under vacuum. Thereto was added trifluoromethanesulfonyl chloride (100 g (0.595 mol)). Airtightly heat up slowly, and stir for 7 hours while the inner temperature of the reactor is 40-50°C. At this time, the reactor pressure was 0.90 MPa. The gas in the reactor was circulated in a condenser cooled to -40°C to -20°C, and collected with a trap cooled by liquid nitrogen. 54.2 g of the captured product was obtained, and the generation of trifluoromethanesulfonyl fluoride was confirmed by gas chromatography (GC) analysis, and it was obtained with a purity of 98.8% and a yield of 81% (conversion rate 99%, selectivity 81%).
[0052] In this way, it can be seen that when the amount of water is reacted in the range of 0.6 mass % to 10.0 mass % with respect to 100 mass % of the metal fluoride, the reaction proceeds well, and the target...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com