Nitroimidazoline derivatives and preparation method thereof and application thereof
A technology for nitroimidazolidine derivatives, which is applied in the field of nitroimidazolidine derivatives and their preparation and application, can solve difficult problems such as treatment, and achieve high insecticidal activity and simple synthesis method
Inactive Publication Date: 2010-09-15
WUHAN INSTITUTE OF TECHNOLOGY
View PDF2 Cites 9 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In addition, because imidacloprid contains a large amount of pollutants in the production process of pyridine ring, it is difficult to control
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
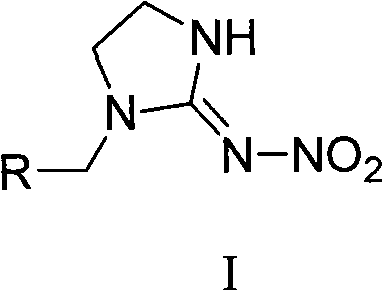
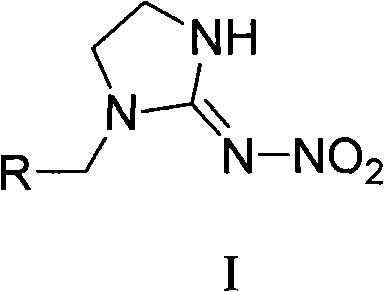
The invention relates to nitroimidazoline derivatives. The nitroimidazoline derivatives have a structural formula shown in a formula I, wherein R is substituted benzene, naphthalene, benzoxazole or benzimidazole. The preparation method comprises that different substituted chloromethylene compounds, N-nitroimidazoline, potassium carbonate and solvent are reacted to form the nitroimidazoline derivatives. The nitroimidazoline derivatives have the advantages that: 1) the synthetic method for the nitroimidazoline derivative compounds is simple; and 2) the nitroimidazoline derivative compounds have a structure different from that of imidacloprid and have high insecticidal activity, the structure of the compounds does not comprise pyridine rings, and the compounds have wide development prospect.
Description
technical field The present invention relates to nitroimidazolidine derivatives and their preparation methods and applications. Background technique Neonicotinoid insecticides, especially imidacloprid, have made great contributions to the control of piercing-sucking aphids, planthoppers, and leafhoppers since they were put on the market in 1991. The development of insecticides that act on nicotinic acetyl receptors is still Has great potential. It can be said that there is currently no crop that does not use imidacloprid, especially the two major crops of rice and vegetables, which are used at least twice a season, effectively solving the long-standing problems of piercing-sucking aphids, planthoppers, and leafhoppers. prevention issues. Imidacloprid is a new type of nitroimine insecticide. Its insecticidal mechanism is mainly a nicotinic acetylcholine receptor agonist in the insect nervous system, thereby blocking the normal conduction of the insect central nervous system...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D233/52C07D413/06C07D403/06A01N51/00A01P7/04
Inventor 巨修练刘根炎卢伦
Owner WUHAN INSTITUTE OF TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com