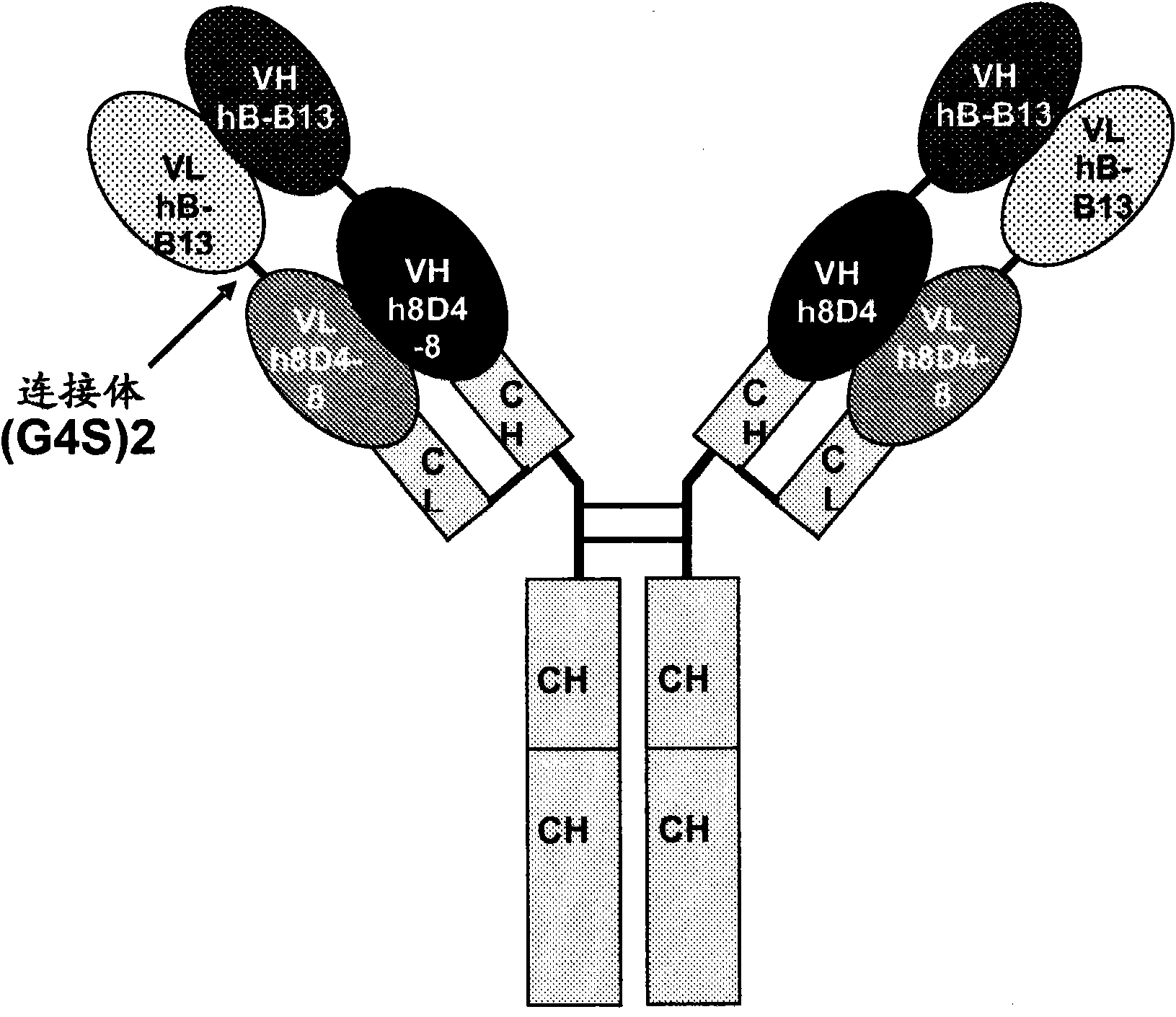
Antibodies that bind IL-4 and/or IL-13 and their uses
A technology of IL-4 and antibodies, applied in the direction of antibodies, antibody medical ingredients, medical preparations of non-active ingredients, etc., can solve the problem of no specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
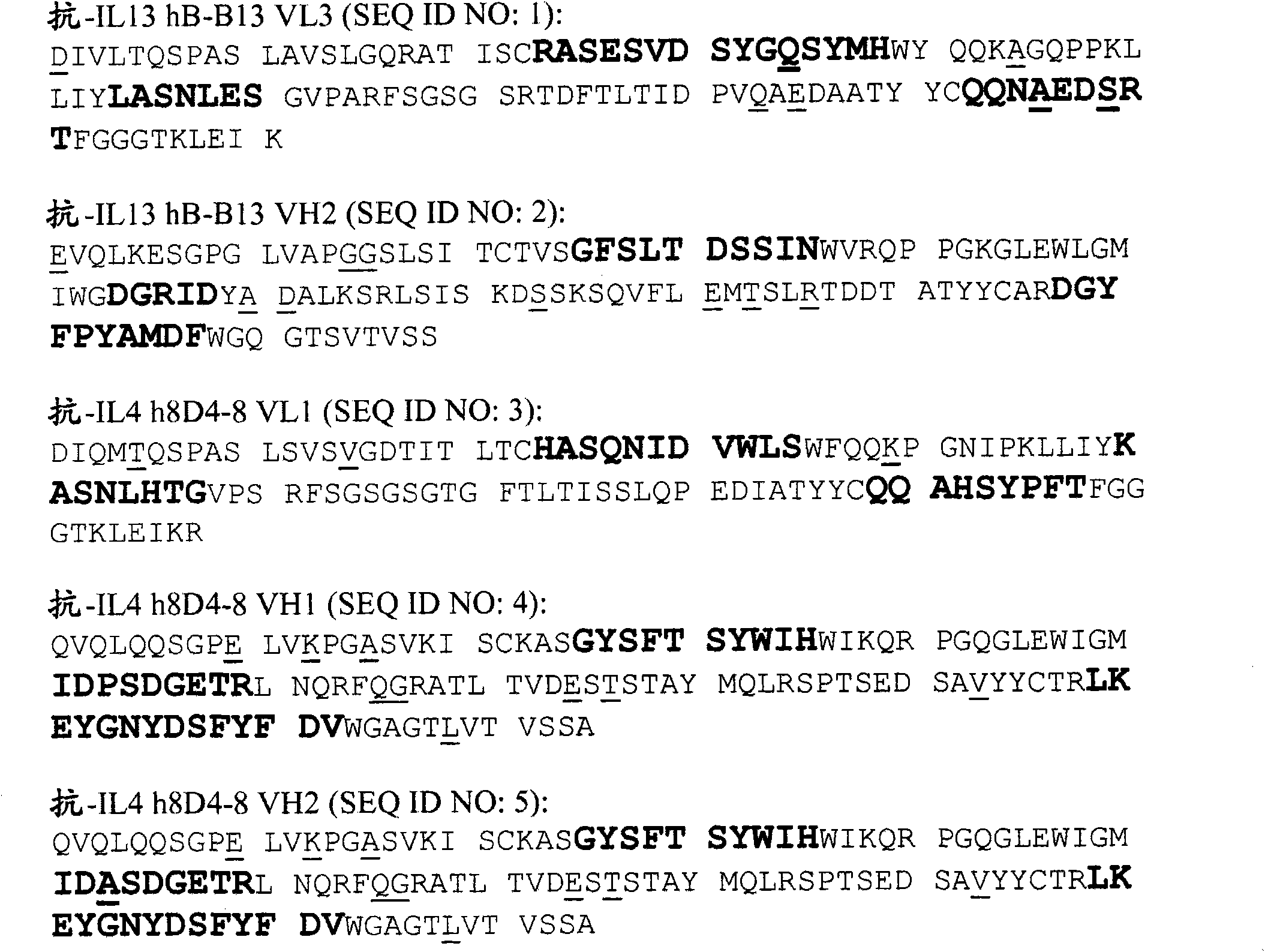
[0288] Example 1: Sequencing of the Fv domain of mouse anti-human IL-13 monoclonal antibody clone B-B13
[0289] The reagent used in the following method was mouse anti-IL-13 monoclonal antibody clone B-B13 purchased from Cell Sciences, Inc. (Canton, MA, USA). Cell Sciences is the US distributor for Diaclone (Besancon, France), maker of antibody B-B13.
[0290] The amino acid sequence of anti-IL-13 monoclonal antibody clone B-B13 was determined using a combination of Edman N-terminal sequencing and mass spectrometry. Antibodies are processed in various ways as described below to produce polypeptides or peptide fragments which are then fractionated in various ways to prepare samples for subsequent Edman N-terminal sequencing and liquid chromatography / mass spectrometry / mass spectrometry (LC-MS / MS ) analysis, which is matched against peptides in related protein sequence databases.
[0291] SDS-Page of antibodies, with or without pyrogluamino-treatment to separate heavy and ligh...
Embodiment 2
[0297] Example 2: Fv domain sequencing of mouse anti-human IL-4 monoclonal antibody clone 8D4-8
[0298] Reagents Mouse anti-IL-4 monoclonal antibody clone 8D4-8 was purchased from Biozol diagnostica Vertrieb GmbH (Eching, Germany). Biozol is the German distributor of BioLegend (San Diego, CA, USA), which produces the 8D4-8 antibody.
[0299] The amino acid sequence of the mouse anti-IL-4 monoclonal antibody (clone 8D4-8) was determined using a combination of Edman sequencing and mass spectrometry (Pham et al., 2006, Anal. Biochem. 352:77-86; Roberts et al., 2005 , Anal. Chem. 67:3613-25). Briefly, antibodies are first separated into light and heavy chains, and then each chain is cleaved with sequence-specific proteases or chemicals. The resulting peptides were separated by reverse phase chromatography and analyzed by matrix-assisted laser desorption / ionization mass spectrometry (MALDI) and / or LC-MS / MS. The unique peptides as well as the complete heavy and light chains are ...
Embodiment 3
[0300] Example 3: Humanization of the Fv domain of mouse anti-human IL-13 monoclonal antibody clone B-B13
[0301] The humanization protocol described here was used to achieve humanization of the B-B13 clone. Six humanized versions were suggested, including mutations in the CDRs to address problematic residues (deamidation sites, solvent-exposed methionines, acid-labile positions).
[0302] The VL&VH sequence of B-B13 was blasted with the protein database (PDB) version in July 2007. Most similar light and heavy chain amino acid sequences were retrieved. The most homologous of the variable light chains was found to be 1EGJ. The most homologous of the variable heavy chains was found to be 1FNS. The 1EGJ & 1FNS structures were used to construct homology models of the variable domains, which were then energy minimized using standard procedures implemented in the Molecular Operating Environment (MOE). MOE is a comprehensive set of computer-aided drug design software provided by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com