Pharmaceutical composition containing idebenone for the treatment of liver disorders
A composition and disease technology, applied in the direction of drug combination, medical preparations containing active ingredients, drug delivery, etc., can solve problems such as unsuitable for injection, low solubility, limited use, etc.
Inactive Publication Date: 2010-08-25
ALPHARX
View PDF5 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
An inclusion complex of idebenone with cyclodextrins has been described, but it is water-dispersible rather than water-soluble and therefore not suitable for injection
The drug precipitates from these emulsions during storage, which limits their use in injectable formulations
Combinations of solvents, oils, and surfactants result in emulsions with relatively large droplet sizes in vivo, making them unsuitable for intravenous administration
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1-10
Embodiment 1
Embodiment 11
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
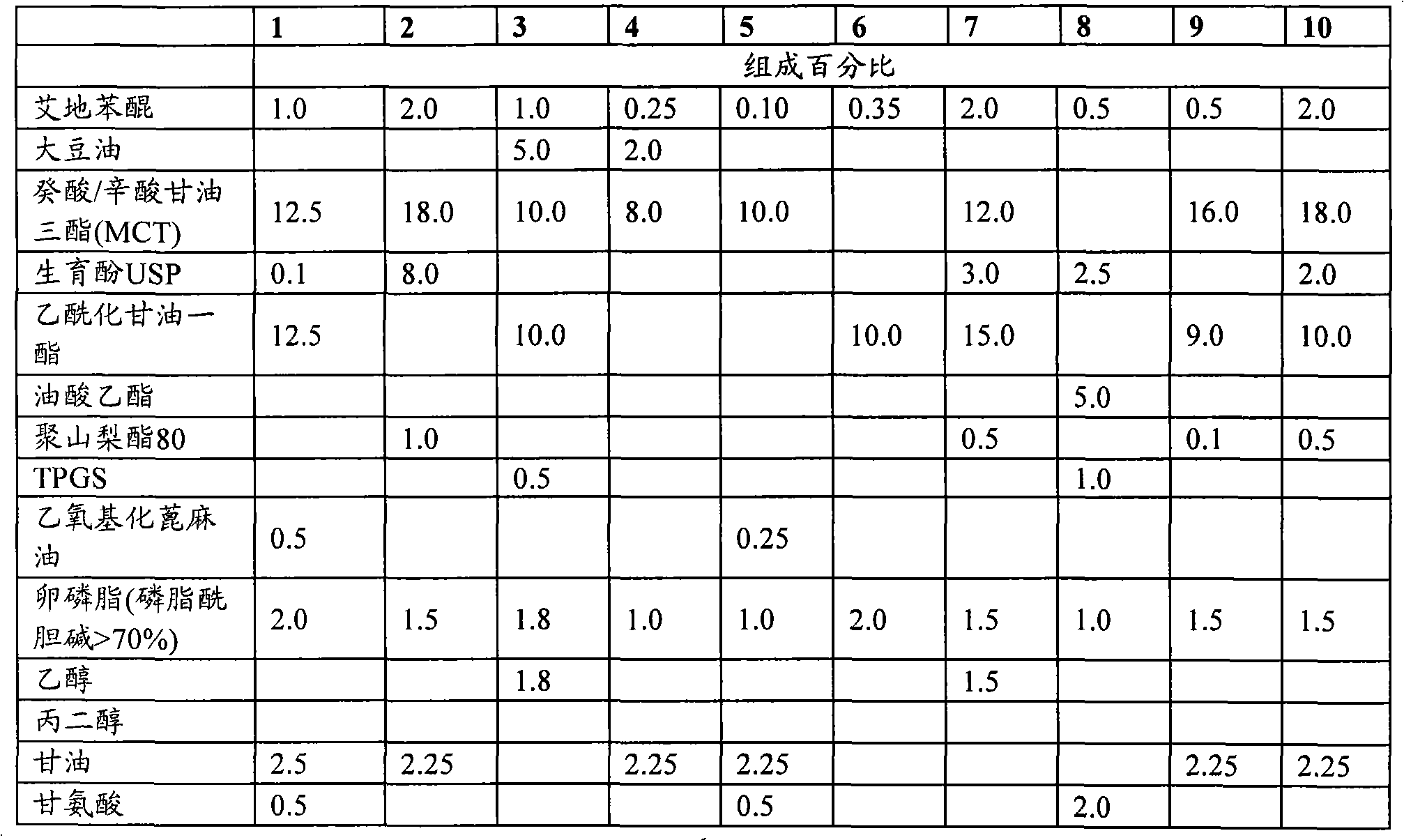
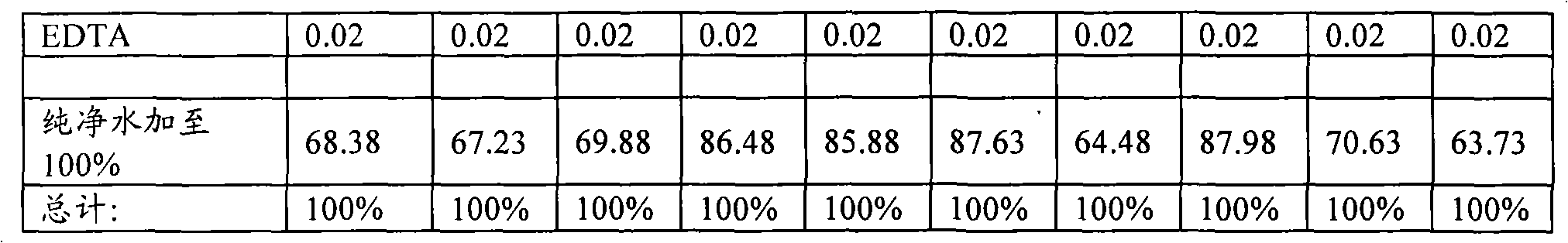
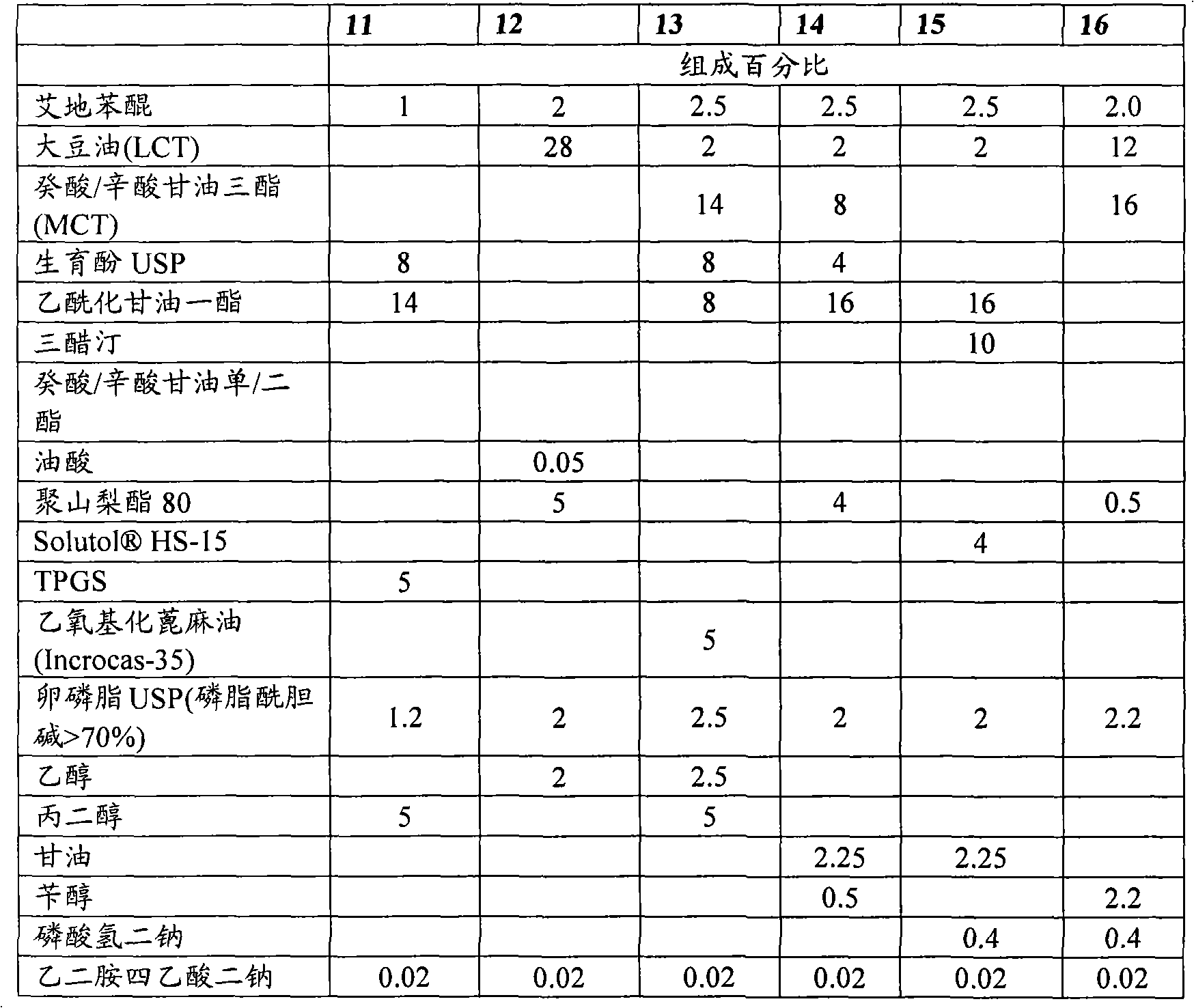
The invention describes the use of an injectable form of Idebenone to protect against hepatic damage, improve recovery from liver trauma, poisoning, vapor intoxication, degenerative diseases, hepatocyte function loss and pathology associated with inflammation or infection. The use of injectable Idebenone restores liver function, suppresses elevated enzyme levels, decreases alcoholic and drug abuse associated syndromes, symptoms of acute hepatitis of various origins, the consequences of liver reperfusion and other signs of liver damage.
Description
technical field The present invention relates to the field of preparing stable formulations of idebenone suitable for parenteral administration. Existing oral dosage forms of idebenone are associated with hypermetabolism in the liver ("first-pass effect") and cannot be administered in acute situations or while the patient is unconscious. It is therefore highly desirable to develop an injectable form of idebenone. Background technique Associated with various diseases, symptoms and many other conditions (acute liver failure, acute hepatitis, elevated hepatic enzymes, liver injury and trauma, liver infarction, cirrhosis , acetaminophen poisoning (paracetamol / acetaminophen poisoning), alcohol intoxication (alcoholic intoxication), post-anesthesia liver damage (post-anesthesia hepatic damage) related to liver injury needs to be effectively treated and prevented [1]. Chemicals often cause subacute liver injury, manifested as abnormal liver enzyme tests, but without obvious clin...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/122A61P1/16
CPCA61K9/0019A61K31/122A61K47/14A61K9/1075A61K9/107A61P1/00A61P1/16
Inventor 约瑟夫·施瓦茨迈克尔·韦斯帕皮尔
Owner ALPHARX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com