Preparation and application of human galectin-9 deletant for enhancement of immune response
A galectin and immunity-enhancing technology, applied in the field of protein preparation, can solve the problems of inconvenient application of bidirectional regulation, and achieve the effect of improving immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1 The acquisition of target gene and the construction of prokaryotic expression system
[0015] 1) Gene transfer
[0016] According to the gene sequence and the restriction site of the PET-32a vector, Sac I and Xho I double restriction sites were used to capture PCR Gal-9C and Gal-9, and the gene primers were designed as follows:
[0017] Gal-9CPs: 5'-ccggaattcttcatcaccacc-3' (SEQ ID NO: 1)
[0018] Gal-9C Pa: 5'-ccgctcgagctatgtctgcacatggg-3' (SEQ ID NO: 2)
[0019] Gal-9 Ps: 5'-ccggaattcgccttcagcggttcccagg-3' (SEQ ID NO: 3)
[0020] Gal-9 Pa: 5'-tccagctgacccatgtgcagacataggg-3' (SEQ ID NO: 4)
[0021] After the primers were synthesized, the cDNA generated by reverse transcription of total RNA extracted from human peripheral blood mononuclear cells was used as a template to capture the above two genes (SEQ ID NO: 5 and SEQ ID NO: 6). The PCR cycle conditions were set as 94°C for 5min; 94°C for 30s, 60°C for 30s, 72°C for 50s, 35 cycles; 72°C for 7min, 4°C hol...
Embodiment 2
[0024] Expression and purification of the protein of embodiment 2
[0025] 1) Culture of bacteria
[0026] Pick a single clone, inoculate it into a large amount of LB liquid medium after activation, place it in a shaker at 37°C for 3 or 4 hours, and induce the expression of the target protein until the OD value of the medium is about 0.6. The induction conditions were Gal-9: room temperature (25° C.), 0.05 mM IPTG, 1 L LB liquid medium; Gal-9C: room temperature (25° C.), 0.1 mM IPTG.
[0027] 2) Protein separation and purification
[0028]After induction for about 12 hours, collect the bacteria (7000rpm, 5mins), resuspend the bacteria expressing the two proteins in PBS, put them in liquid nitrogen to freeze and thaw two or three times, and then add a small amount of lysozyme (4°C, 1 hour) try to lyse the bacteria, and finally ultrasonically disrupt (10 minutes each time, twice in total). Centrifuge (10000rpm, 10mins) to take the supernatant and filter it, then apply it to a...
Embodiment 3
[0029] Example 3 Protein function verification
[0030] 1) Gal-9 and Gal-9C pro-inflammatory detection
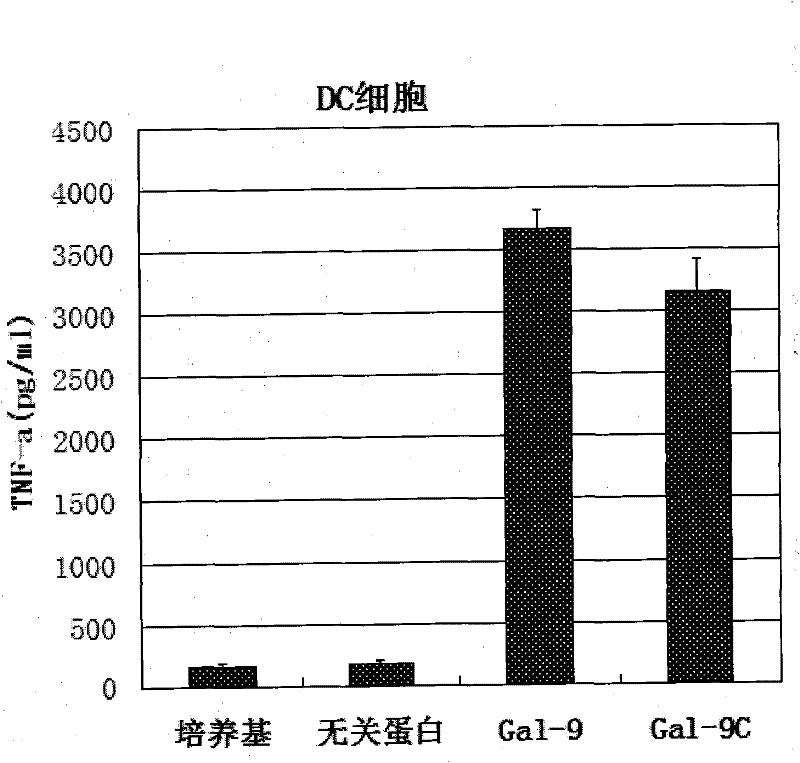
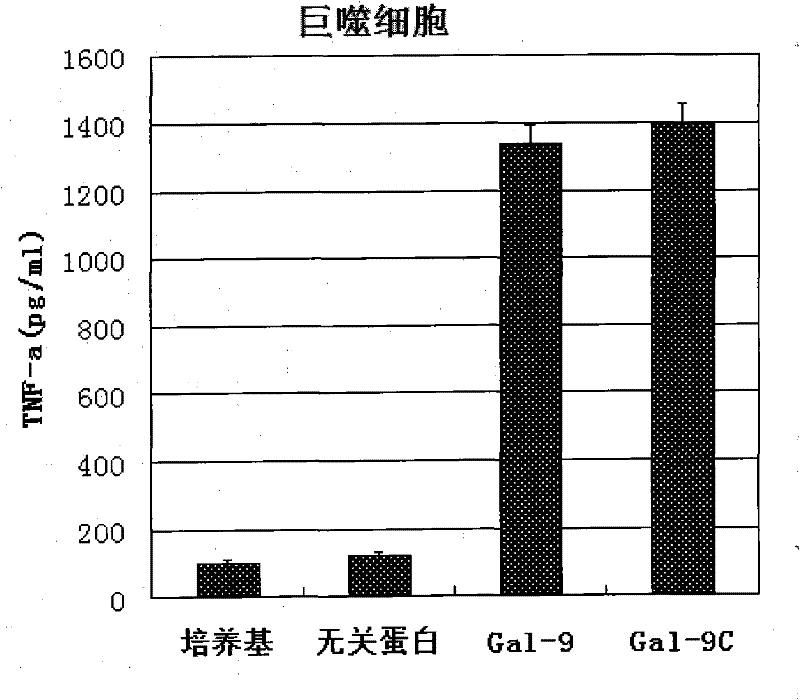
[0031] Human DC cells and macrophages were respectively incubated with samples containing Gal-9 protein and samples containing Gal-9C protein at 37°C for 12-24 hours at different concentrations, and the cell culture supernatant was collected, and TNF-a was detected by ELISA Secretion. ELISA results such as figure 1 and figure 2 : Both Gal-9 and Gal-9C can promote the secretion of inflammatory factors.
[0032] 2) Detection of T cell apoptosis induced by Gal-9 and Gal-9C
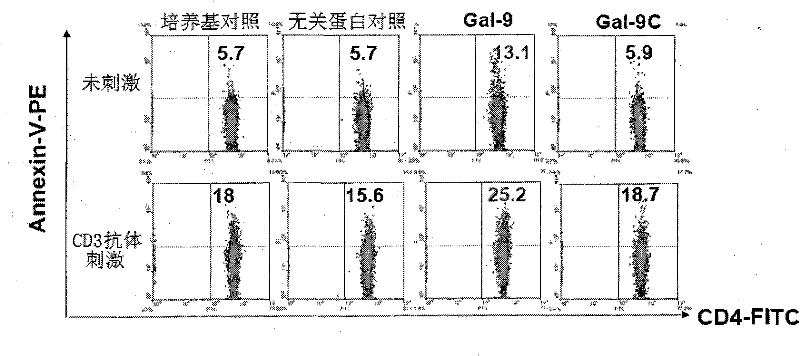
[0033] After co-incubating human lymphocytes with Gal-9 and Gal-9C for 4 hours, the cells were stained with Annexin V-PE and CD4-FITC to detect CD4 + Apoptosis of T cells. The flow detection results are as follows: image 3 : Gal-9 significantly induces CD4 + T cell apoptosis.
[0034] The above two results show that Gal-9 can both enhance immunity and suppress immunity; while Gal-9C only retains...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com