High-purity Febuxostat and preparation method thereof
A febuxostat, high-purity technology, applied in the field of medicinal chemistry, can solve problems such as impurities that are not easy to reduce or remove
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
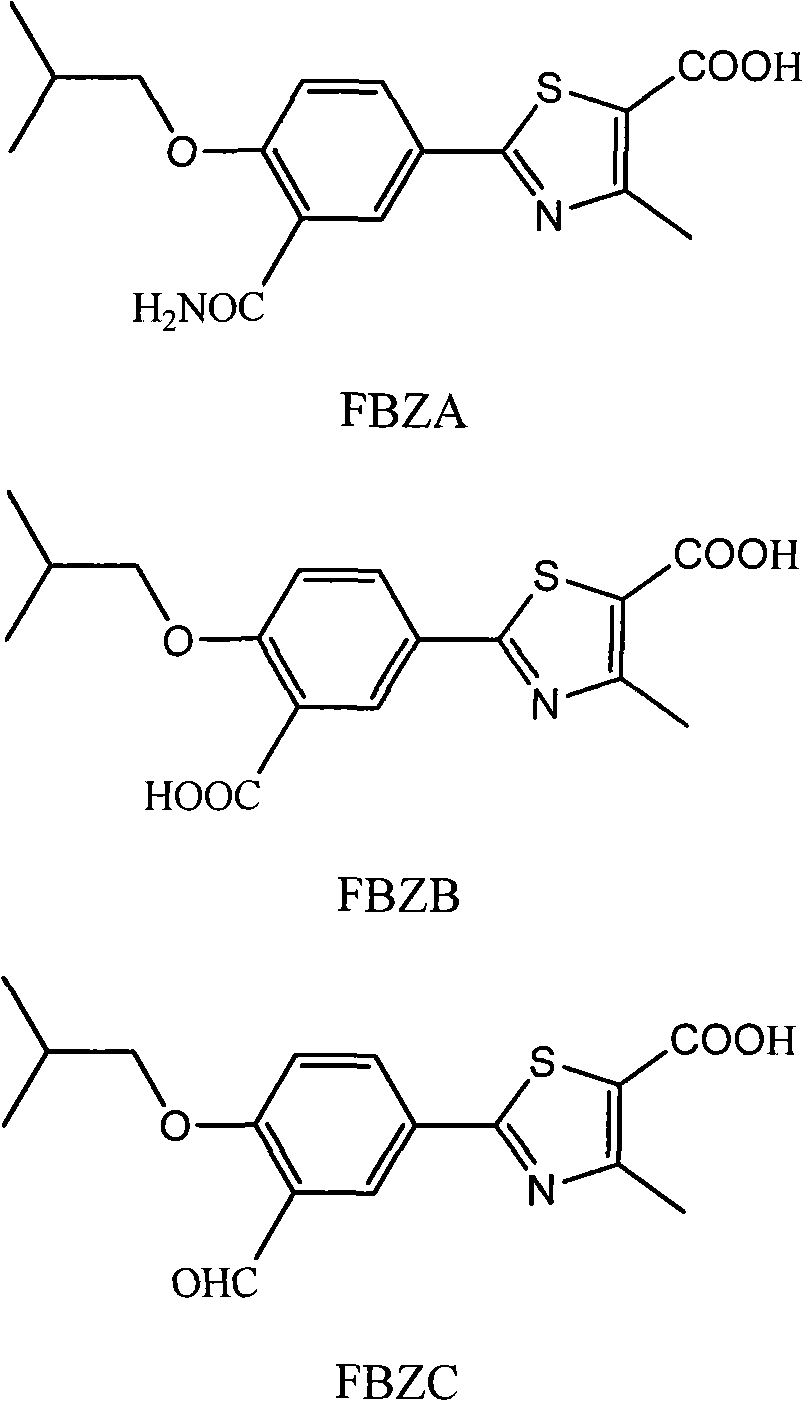
[0054] Preparation of 2-[3-carbamoyl-4-(2-methylpropoxy)phenyl]-4-methyl-5-thiazolecarboxylic acid (FBZA)
[0055] In a 500ml three-necked flask, add 20.0g of ethyl 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methyl-5-thiazolecarboxylate, 10% hydrogen Dissolve 200ml of sodium oxide and 70ml of tetrahydrofuran, stir and react at 75-80°C for about 24 hours, cool, adjust the pH to about 3 with concentrated hydrochloric acid, and precipitate a white solid, filter, wash the filter cake with water, recrystallize with methanol, and collect by filtration Precipitate, the filter cake was dried under reduced pressure (-0.085~-0.090MPa) at 80~85°C to obtain 2-[3-carbamoyl-4-(2-methylpropoxy)phenyl]-4- Methyl-5-thiazolecarboxylic acid (FBZA) 9.4g, white crystal. HPLC purity: 98%. IR (KBr): 3464, 3399, 3190, 2963, 1693, 1646, 1598, 1505, 1411, 1256, 1161, 1016cm -1 . 1 H-NMR (500MHz, DMSO-d 6 )δ(ppm): 8.342~8.346(1H,d), 8.013~8.035(1H,m), 7.255~7.273(1H,d), 3.972~3.985(2H,d), 2.095~2.148(...
Embodiment 2
[0057] Preparation of 2-[3-carboxy-4-(2-methylpropoxy)phenyl]-4-methyl-5-thiazolecarboxylic acid (FBZB)
[0058]In a 500ml three-necked bottle, 22.0g of ethyl 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methyl-5-thiazolecarboxylate, 10% sodium hydroxide Dissolve 220ml and 80ml tetrahydrofuran, heat to vigorous reflux, react for about 48 hours, stop heating, cool, slowly add concentrated hydrochloric acid dropwise, adjust pH to about 3, precipitate solid, filter with suction, wash the filter cake with water to neutral, and pump Dry. The filter cake was recrystallized with absolute ethanol, suction filtered, and the filter cake was dried under reduced pressure (-0.090~-0.095MPa) at 75-85°C to obtain 2-[3-carboxy-4-(2-methylpropoxy) Phenyl]-4-methyl-5-thiazolecarboxylic acid (FBZB) 8.7g, off-white solid. HPLC purity: 98%. IR (KBr): 3396, 2959, 2874, 1692, 1604, 1508, 1422, 1377, 1293, 1252, 1223, 1167, 1111, 1092, 1017, 825cm -1 . 1 H-NMR (500MHz, DMSO-d 6 )δ(ppm): 8.185~8.181(...
Embodiment 3
[0060] Preparation of 2-[3-formyl-4-(2-methylpropoxy)phenyl]-4-methyl-5-thiazolecarboxylic acid (FBZC)
[0061] In a 500ml three-necked flask, add 28.0 g of ethyl 2-[3-formyl-4-(2-methylpropoxy)phenyl]-4-methyl-5-thiazolecarboxylate, 10% hydrogen Dissolve 280ml of sodium and 90ml of ethanol, stir and react at about 80°C for about 4 hours, stop the reaction, cool, slowly add hydrochloric acid dropwise, adjust the pH to 3, precipitate a white solid, filter, wash the filter cake with water, and drain. The filter cake is recrystallized with ethyl acetate, filtered, and the filter cake is dried under reduced pressure (-0.080~-0.085MPa) at 70~75°C to obtain 2-[3-formyl-4-(2-methylpropoxy) 11.2 g of phenyl]-4-methyl-5-thiazolecarboxylic acid (FBZC), white crystal. HPLC purity: 99%. IR (KBr): 3432, 2966, 2871, 1679, 1652, 1605, 1513, 1447, 1427, 1371, 1179, 1111, 1014cm -1 . 1 H-NMR (500MHz, DMSO-d 6 )δ(ppm): 13.360(1H, s), 10.397(1H, s), 8.191~8.153(2H, m), 7.337~7.319(1H,d), 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com