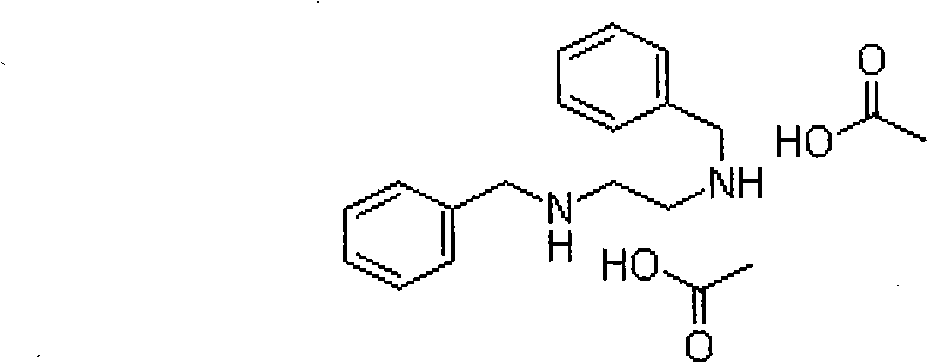
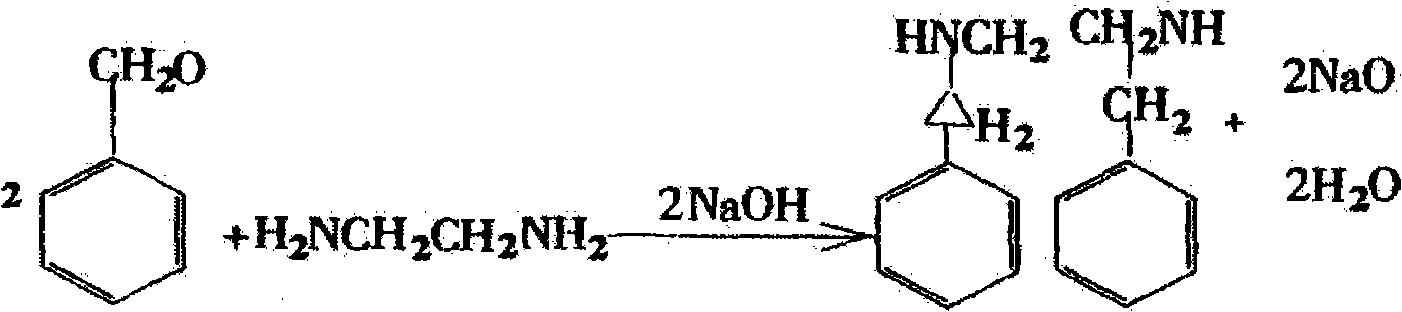
Preparation methods of dibenzyl ethylenediamine and acetate thereof
A technology of dibenzylethylenediamine acetate and dibenzylethylenediamine, which is applied in the field of compound preparation, can solve the problems of large waste water discharge, unsuitability for large-scale production, large unit consumption of benzaldehyde, etc. The effect of convenience, reduced labor intensity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Add 240ml of ethanol and 400g of benzaldehyde into a three-necked reaction flask, stir to raise the temperature to 45°C, add 120g of ethylenediamine dropwise, after the dropwise addition, keep the reaction at 40-60°C for 1-1.5 hours, then cool down to 10°C, Filter to get the Schiff base. Dissolve all the Schiff base obtained in the previous reaction into 240ml of ethanol, add it to a 1000L hydrogenation reactor, then add 4g of palladium carbon with a palladium content of 5-10%, control the hydrogen pressure at 5-10kPa, stir and heat up to 60 ℃, react with hydrogen until no hydrogen is absorbed. The solvent after the hydrogenation reaction is completed is filtered to obtain an ethanol solution of dibenzylethylenediamine. The ethanol solution of dibenzylethylenediamine was distilled to dryness, and the ethanol solvent was removed to obtain dibenzylethylenediamine solid. Alternatively, filter the dibenzylethylenediamine ethanol solution after the hydrogenation...
Embodiment 2
[0042] Example 2: Add 240ml of methanol and 400g of benzaldehyde into a three-necked reaction flask, stir and raise the temperature to 50°C, add 120g of ethylenediamine dropwise, after the dropwise addition, keep the reaction at 40-60°C for 1-1.5 hours, then cool down to 6°C, Filter to get the Schiff base. Dissolve all the Schiff base obtained in the previous reaction into 240ml of methanol, add it to a 1000L hydrogenation reactor, then add 4g of palladium carbon with a palladium content of 5-10%, control the hydrogen pressure at 5-10kPa, stir and heat up to 65 ℃, react with hydrogen until no hydrogen is absorbed. The solvent after the hydrogenation reaction is completed is filtered to obtain a methanol solution of dibenzylethylenediamine. The methanol solution of dibenzylethylenediamine was distilled to dryness, and the methanol solvent was removed to obtain dibenzylethylenediamine as a solid. Alternatively, filter the dibenzylethylenediamine methanol solution after the hyd...
Embodiment 3
[0043] Example 3: Add 240ml of ethanol and 400g of benzaldehyde into a three-necked reaction flask, stir and raise the temperature to 55°C, add 120g of ethylenediamine dropwise, after the dropwise addition, keep the reaction at 40-60°C for 1-1.5 hours, then cool down to 8°C, Filter to get the Schiff base. Dissolve all the Schiff base obtained in the previous reaction in 240ml of ethanol, add it to a 1000L hydrogenation reactor, then add 12g of Raney nickel, control the hydrogen pressure at 5-10kPa, stir and raise the temperature to 70°C, and let the hydrogen react until no until the hydrogen is absorbed. The solvent after the hydrogenation reaction is completed is filtered to obtain an ethanol solution of dibenzylethylenediamine. The ethanol solution of dibenzylethylenediamine was distilled to dryness, and the ethanol solvent was removed to obtain dibenzylethylenediamine solid. Alternatively, filter the dibenzylethylenediamine ethanol solution after the hydrogenation reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com