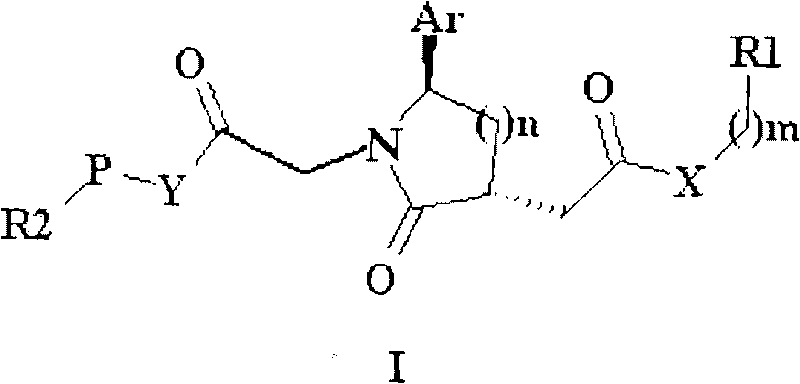
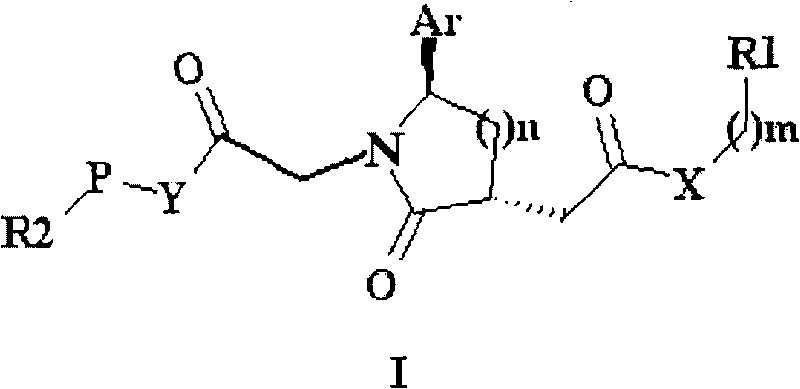
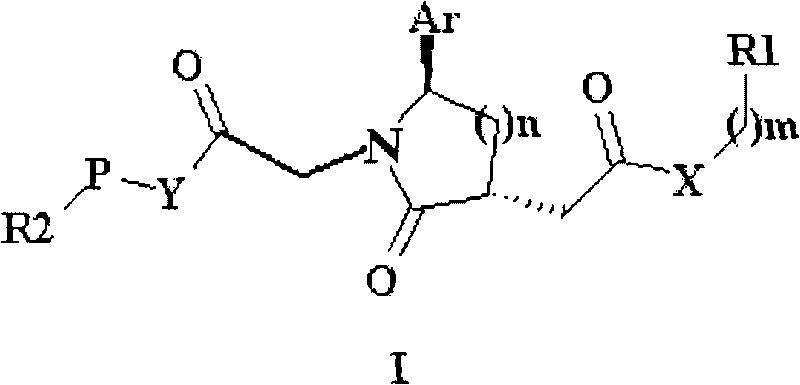
Trisubstituted chiral lactam derivative and preparation method and application thereof
A lactam and substituent technology, applied in the field of tri-substituted chiral γ-lactam derivatives, can solve the problems of ineffectiveness of disease development and potential safety hazards.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0145] The present invention is further illustrated below through specific intermediates and examples, but it should be understood that these intermediates and examples are only used for more detailed description, and should not be interpreted as being used to limit the present invention in any form. invention.
[0146] The present invention provides general and / or specific descriptions of the materials and test methods used in the tests. While many of the materials and methods of manipulation which are employed for the purposes of the invention are well known in the art, the invention has been described here in as much detail as possible. It will be clear to those skilled in the art that in the following, unless otherwise specified, the materials and operation methods used in the present invention are well known in the art.
[0147] The melting points of the compounds were determined by a YRT-3 melting point apparatus, and the temperature was not corrected. 1 The H-NMR spec...
Embodiment 1
[0222] Example 1 : α(R)-{[(2-methyl-3-chloro)phenyl]carbamoylmethyl}-γ(R)-(4-bromophenyl)-N-{[3-(2- Oxo-pyrrolidin-1-yl)propyl]carbamoylmethyl}-γ-lactam
[0223]
[0224] Dissolve intermediate 20 (300mg, 0.625mmol) in 20mL of anhydrous dichloromethane, add carbonyldiimidazole (CDI, 122mg, 0.75mmol), stir at room temperature for 2-3 hours, then add 1-(3- Aminopropyl)pyrrolidin-2-one (84mg, 0.594mmol), stirred overnight at room temperature. After the reaction was completed, saturated brine was added thereto for extraction, and the combined organic phases were dried (Na 2 SO 4 ), filtered, concentrated and separated by column (eluent: dichloromethane / methanol / ammonia water system) to obtain a white solid product.
[0225] 1 H-NMR (400MHz, CDCl 3 )δppm: 8.62 (1H, s), 7.58 (1H, d, J = 7.56Hz), 7.49 (3H, m), 7.20 (1H, d, J = 7.84Hz), 7, 12 (1H, m), 7.05(2H, m), 4.78(1H, d, J=7.29Hz), 4.44(1H, d, J=16.53Hz), 3.14-3.45(8H, brm), 2.98(1H, m), 2.87(1H , m), 2.59 (1H, m), 2.0...
Embodiment 2
[0226] Example 2 : α(R)-{[(2-methyl-3-chloro)phenyl]carbamoylmethyl}-γ(R)-(4-bromophenyl)-N-[(1-phenylpiper Pyridin-4-yl)carbamoylmethyl]-γ-lactam
[0227]
[0228] Starting from intermediate 20 and 1-benzyl-4-aminopiperidine, the title compound was prepared by the same operation as described in Example 1 to obtain a white solid product.
[0229] 1 H-NMR (400MHz, CDCl 3 )δppm: 7.56 (2H, m), 7.49 (2H, m), 7.27-7.35 (5H, brm), 6.99-7.11 (5H, brm), 4.66 (1H, dd, J=9.52, 3.08Hz), 4.47 (1H, d, J=16.75Hz), 3.70(1H, m), 3.44(2H, s), 3.16(1H, m), 3.11(1H, d, J=16.75Hz), 2.98(1H, m) , 2.74-2.79(3H, brm), 2.52(1H, m), 2.32(3H, s), 1.94-2.15(3H, brm), 1.37-1.80(4H, brm); FAB-MS(m / z) : 653.0[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com