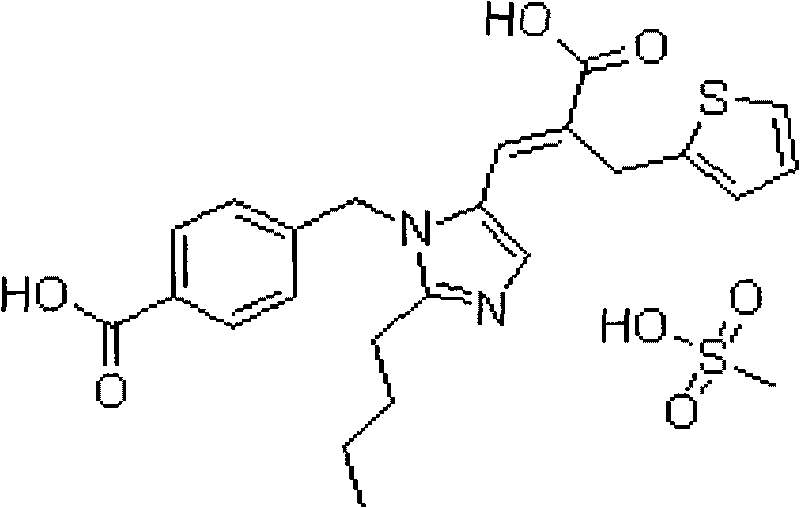
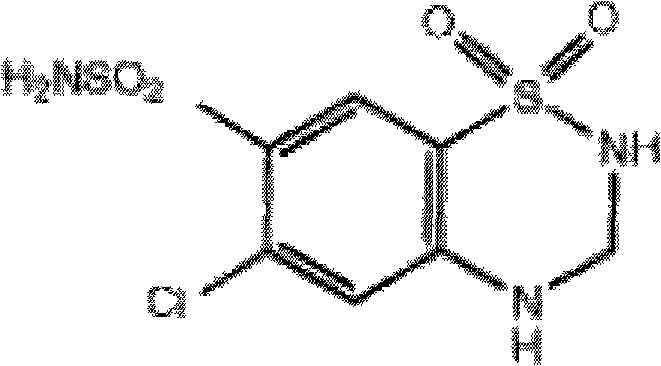
Pharmaceutical composition for treating cardiovascular diseases and preparation method and use thereof
A composition and cardiovascular technology, applied in the direction of cardiovascular system diseases, drug combinations, pharmaceutical formulas, etc., can solve undiscovered problems, reduce adverse reactions, improve antihypertensive effect, and reduce the number of times of taking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1 eprosartan amlodipine hydrochlorothiazide film-coated tablet (300 / 5 / 25mg)
[0085] prescription:
[0086] Component Dosage
[0087] Amlodipine 5.0g
[0088] Hydrochlorothiazide 25.0g
[0089] Eprosartan 300.0g
[0090] Microcrystalline Cellulose 150.0g
[0091] Lactose 60.0g
[0092] Povidone 25.0g
[0093] Magnesium Stearate 5.0g
[0094] Opadry Appropriate amount
[0095] 5% starch slurry appropriate amount
[0096] A total of 1000 pieces were made
[0097] Preparation:
[0098] Pass the above prescription amount of the principal ingredient and auxiliary materials through an 80-mesh sieve, and then mix the prescription amount of amlodipine, hydrochlorothiazide, and eprosartan evenly by equal-volume incremental method. Then mix the well-mixed main ingredient with other auxiliary materials and 70% povidone evenly, make soft material with prepared 5% starch slurry, pass through 18 mesh sieve and granulate, dry at 60°C for 3 hours, and use 22 mesh ...
Embodiment 2
[0100] Embodiment 2 eprosartan amlodipine hydrochlorothiazide film-coated tablet (400 / 5 / 25mg)
[0101] prescription:
[0102] Component Dosage
[0103] Amlodipine 5.0g
[0104] Hydrochlorothiazide 25.0g
[0105] Eprosartan 400.0g
[0106] Microcrystalline Cellulose 200.0g
[0107] Lactose 80.0g
[0108] Sodium carboxymethyl starch 40.0g
[0109] Magnesium Stearate 8.0g
[0110] Opadry Appropriate amount
[0111] 5% starch slurry appropriate amount
[0112] A total of 1000 pieces were made
[0113] Preparation:
[0114] Pass the above prescription amount of the principal ingredient and auxiliary materials through an 80-mesh sieve respectively, and then mix the prescription amount of amlodipine and hydrochlorothiazide eprosartan evenly by the method of equal addition. Then the well-mixed main ingredient is mixed evenly with other auxiliary materials and 70% carboxymethyl starch sodium, and the soft material is made of 5% starch slurry prepared, granulated through a 18...
Embodiment 3
[0116] Embodiment 3 eprosartan amlodipine hydrochlorothiazide film-coated tablet (600 / 5 / 12.5mg)
[0117] prescription:
[0118] Component Dosage
[0119] Amlodipine 5.0g
[0120] Hydrochlorothiazide 12.5g
[0121]Eprosartan 600.0g
[0122] Microcrystalline Cellulose 200.0g
[0123] Pregelatinized starch 120.0g
[0124] Povidone 40.0g
[0125] Magnesium Stearate 8.0g
[0126] Opadry Appropriate amount
[0127] 5% PVP-K30 appropriate amount
[0128] A total of 1000 pieces were made
[0129] Preparation:
[0130] Pass the above prescription amount of the principal ingredient and auxiliary materials through an 80-mesh sieve, and then mix the prescription amount of amlodipine, hydrochlorothiazide, and eprosartan evenly by equal-volume incremental method. Then the well-mixed main ingredient is mixed evenly with other auxiliary materials and 70% povidone, and the prepared soft material is made of 5% PVP-K30, granulated through a 18-mesh sieve, dried at 60°C for 3 hours, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com