Preparation method of 5,6,7,4'-tetramethoxy flavones of scutellarin and aglucone key intermediate thereof
A technology of tetramethoxyflavone and scutellarin, which is applied in the field of compound preparation, can solve problems such as complicated operation requirements, and achieve the effects of simple operation steps, good application value and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
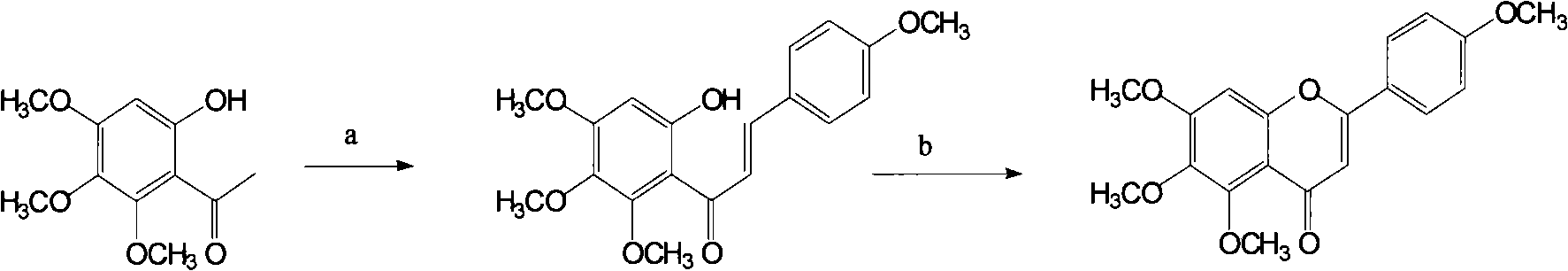
[0017] Embodiment 1: the preparation of chalcone (compound 3)
[0018] Weigh 11.3g (0.05mol) of the compound 2,2-hydroxy-4,5,6-trimethoxyacetophenone and 7.4g (0.06mol) of p-methoxybenzaldehyde in a 250ml round bottom flask, add 150ml of methanol and 22.5 g of solid KOH, stirred and reacted at room temperature for 36 hours. After most of the methanol was distilled off under reduced pressure, 200ml of water was added, and then the pH was adjusted to 3-4 with concentrated hydrochloric acid. Precipitate a red precipitate, put it well and filter it, wash the precipitate with a little water, press dry, and dry at 60°C. Example 1 is the first step reaction of the present invention to prepare scutellarin and its aglycone key intermediate 5,6,7,4'-tetramethoxyflavone, which is obtained from 2-hydroxy-4,5,6- The chalcone derivative (compound 3) was prepared by condensation of trimethoxyacetophenone and p-methoxybenzaldehyde. The first step was reacted to obtain 14.5 g of compound 3 a...
Embodiment 2
[0019] Embodiment 2: the preparation of 5,6,7,4'-tetramethoxyflavone (compound 1)
[0020] Weigh 17.2g (0.05mol) of compound A9 into a 250ml round bottom flask, add 50ml of DMSO and a little iodine, heat to reflux for 30min, then let the reaction solution cool down, add 100ml of cold water, stir well, and filter to obtain the compound A7 is off-white crystal. Example 2 is the second step reaction of the present invention to prepare scutellarin and its aglycone key intermediate 5,6,7,4'-tetramethoxyflavone, which is derived from the chalcone prepared in Example 1 5,6,7,4'-tetramethoxyflavone was obtained after oxidative cyclization of compound (compound 3). In the second step, 14.6 g of dry product of compound 1 was obtained, and the reaction yield was about 85%. 1 HNMR (CDCl 3 ): δ7.83(d, J=9.0Hz, 2H), δ7.01(d, J=9.0Hz, 2H), δ6.81(s, 1H), δ6.59(s, 1H), δ4. 00(s, 3H), δ3.99(s, 3H), δ3.93(s, 3H), δ3.89(s, 3H). MS (m / e): 342.
[0021] The above two examples illustrate the tw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com