Preparation method of inactivated vaccine of infectious coryza of chicken
A technology for chicken infectious rhinitis and inactivated vaccine, which is applied in the field of inactivated vaccine preparation, can solve the problems of complex process, low yield and high production cost, and achieves the effects of increasing the number of cultured bacteria, reducing production cost, and being easy and feasible to operate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1. Preparation of primary slant strains: dilute Haemophilus paragallinarum strains and inoculate chicken agar plates at 37°C for 22 hours, select 10 typical colonies to inoculate chicken agar slant medium, and cultivate at 37°C for 24 hours to obtain primary slant strains. Place in a 4°C refrigerator for later use;
[0022] 2. Preparation of secondary strains: Inoculate the primary slant strains into the chicken broth culture medium and incubate at 37°C for 12 hours in a full-temperature shaker. After the pure test confirms the sterility, obtain the secondary strains for future use;
[0023] 3. Bacterial liquid culture: inoculate the secondary strains into semi-synthetic medium and cultivate them in a biological fermentation tank; culture temperature: 37°C, rotation speed: 100r / min, culture time 8h; count live bacteria on the sampling plate and confirm that there is no After sterilizing, it is inactivated, and it is obtained.
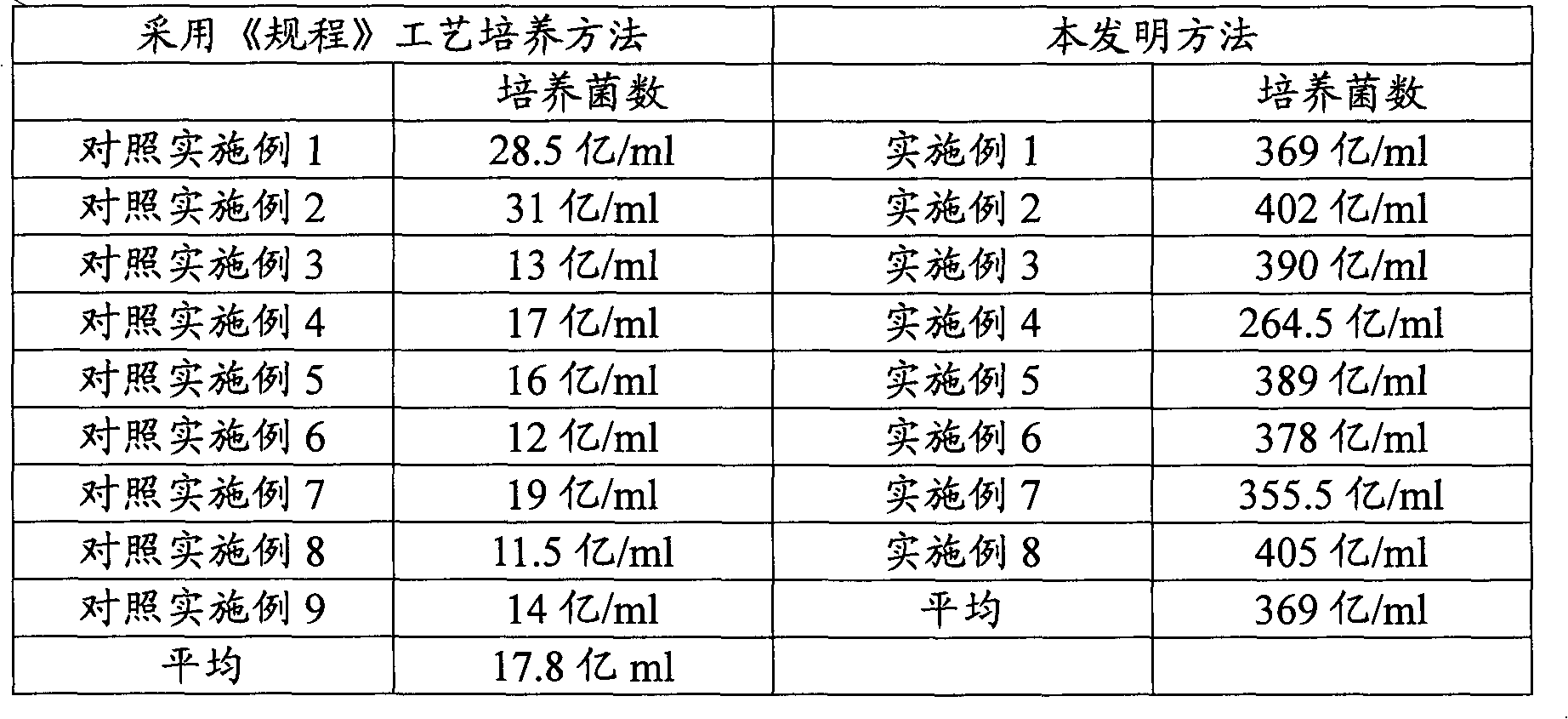
[0024] Using the "Regulations for Veterin...
Embodiment 2
[0026] 1. Preparation of primary slant strains: dilute Haemophilus paragallinarum strains and inoculate chicken agar plates at 37°C for 24 hours, select 20 typical colonies to inoculate chicken agar slant medium, and cultivate at 37°C for 24 hours to obtain primary slant strains. Place in a 4°C refrigerator for later use;
[0027] 2. Preparation of secondary strains: Inoculate the primary slant strains into the chicken broth culture medium and incubate at 37°C for 12 hours in a full-temperature shaker. After the pure test confirms the sterility, obtain the secondary strains for future use;
[0028] 3. Bacterial liquid culture: Inoculate the secondary strains into semi-synthetic medium for cultivation in a biological fermentation tank; cultivation temperature: 37°C, rotational speed: 200r / min, cultivation time 8h; The method of large ventilation volume; after taking a sample plate and counting the live bacteria to confirm the sterility, it is inactivated to obtain the product. ...
Embodiment 3
[0031] 1. Preparation of primary slant strains: dilute Haemophilus paragallinarum strains and inoculate chicken agar plates at 37°C for 23 hours, select 15 typical colonies to inoculate chicken agar slant medium, and cultivate at 37°C for 24 hours to obtain primary slant strains. Place in a 4°C refrigerator for later use;
[0032] 2. Preparation of secondary strains: Inoculate the primary slant strains into the chicken broth culture medium and incubate at 37°C for 12 hours in a full-temperature shaker. After the pure test confirms the sterility, obtain the secondary strains for future use;
[0033] 3. Bacterial liquid culture: inoculate the secondary strains into semi-synthetic medium and cultivate them in a biological fermentation tank; culture temperature: 37°C, rotation speed: 150r / min, culture time 8h; count live bacteria on the sampling plate and confirm that there is no After sterilizing, it is inactivated, and it is obtained.
[0034] Using the "Regulations for Veterin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com